Abstract
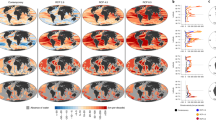
Marine fishes and invertebrates respond to ocean warming through distribution shifts, generally to higher latitudes and deeper waters. Consequently, fisheries should be affected by ‘tropicalization’ of catch1,2,3,4 (increasing dominance of warm-water species). However, a signature of such climate-change effects on global fisheries catch has so far not been detected. Here we report such an index, the mean temperature of the catch (MTC), that is calculated from the average inferred temperature preference of exploited species weighted by their annual catch. Our results show that, after accounting for the effects of fishing and large-scale oceanographic variability, global MTC increased at a rate of 0.19 degrees Celsius per decade between 1970 and 2006, and non-tropical MTC increased at a rate of 0.23 degrees Celsius per decade. In tropical areas, MTC increased initially because of the reduction in the proportion of subtropical species catches, but subsequently stabilized as scope for further tropicalization of communities became limited. Changes in MTC in 52 large marine ecosystems, covering the majority of the world’s coastal and shelf areas, are significantly and positively related to regional changes in sea surface temperature5. This study shows that ocean warming has already affected global fisheries in the past four decades, highlighting the immediate need to develop adaptation plans to minimize the effect of such warming on the economy and food security of coastal communities, particularly in tropical regions6,7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cheung, W. W. L. et al. Climate change induced tropicalization of marine communities in Western Australia. Mar. Freshw. Res. 63, 415–427 (2012)
Wernberg, T. et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nature Clim. Change 3, 78–82 (2013)
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (2005)
Dulvy, N. K. et al. Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. J. Appl. Ecol. 45, 1029–1039 (2008)
Belkin, I. M. Rapid warming of large marine ecosystems. Prog. Oceanogr. 81, 207–213 (2009)
Allison, E. H. et al. Vulnerability of national economies to the impacts of climate change on fisheries. Fish Fish. 10, 173–196 (2009)
Sumaila, U. R., Cheung, W. W. L., Lam, V. W. Y., Pauly, D. & Herrick, S. Climate change impacts on the biophysics and economics of world fisheries. Nature Clim. Change 1, 449–456 (2011)
Simpson, S. D. et al. Continental shelf-wide response of a fish assemblage to rapid warming of the sea. Curr. Biol. 21, 1565–1570 (2011)
Edwards, M. & Richardson, A. J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884 (2004)
Cheung, W. W. L. et al. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nature Clim. Change 3, 254–258 (2013)
Harley, C. D. G. Climate change, keystone predation, and biodiversity loss. Science 334, 1124–1127 (2011)
Cheung, W. W. L. et al. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Change Biol. 16, 24–35 (2010)
Blanchard, J. et al. Potential consequences of climate change for primary production and fish production in large marine ecosystems. Phil. Trans. R. Soc. B 367, 2979–2989 (2012)
Pinsky, M. & Fogarty, M. Lagged social-ecological responses to climate and range shifts in fisheries. Clim. Change 115, 883–891 (2012)
Cheung, W. W. L., Pinnegar, J., Merino, G., Jones, M. C. & Barange, M. Review of climate change impacts on marine fisheries in the UK and Ireland. Aquat. Conserv. Mar. Freshwat. Ecosyst. 22, 368–388 (2012)
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. Lond. B 278, 1823–1830 (2011)
Pörtner, H. O. & Farrell, A. P. Physiology and climate change. Science 322, 690–692 (2008)
Pörtner, H. O. & Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97 (2007)
Ben Rais Lasram, F. et al. The Mediterranean Sea as a ‘cul-de-sac’ for endemic fishes facing climate change. Glob. Change Biol. 16, 3233–3245 (2010)
Collie, J. S., Wood, A. D. & Jeffries, H. P. Long-term shifts in the species composition of a coastal fish community. Can. J. Fish. Aquat. Sci. 65, 1352–1365 (2008)
McGowan, J. A., Cayan, D. R. & Dorman, L. M. Climate-ocean variability and ecosystem response in the northeast Pacific. Science 281, 210–217 (1998)
Pauly, D. et al. Towards sustainability in world fisheries. Nature 418, 689–695 (2002)
Costello, C. et al. Status and solutions for the world’s unassessed fisheries. Science 338, 517–520 (2012)
Howell, P. & Auster, P. J. Phase shift in an estuarine finfish community associated with warming temperatures. Mar. Coast. Fish. 4, 481–495 (2012)
Cheung, W. W. L., Watson, R., Morato, T., Pitcher, T. J. & Pauly, D. Intrinsic vulnerability in the global fish catch. Mar. Ecol. Prog. Ser. 333, 1–12 (2007)
Watson, R. & Pauly, D. Systematic distortions in world fisheries catch trends. Nature 414, 534–536 (2001)
Watson, R., Kitchingman, A., Gelchu, A. & Pauly, D. Mapping global fisheries: sharpening our focus. Fish Fish. 5, 168–177 (2004)
Jones, M., Dye, S., Pinnegar, J., Warren, R. & Cheung, W. W. L. Modelling commercial fish distributions: prediction and assessment using different approaches. Ecol. Modell. 225, 133–145 (2012)
Röckmann, C., Dickey-Collas, M., Payne, M. R. & van Hal, R. Realized habitats of early-stage North Sea herring: looking for signals of environmental change. ICES J. Mar. Sci. 68, 537–546 (2011)
Watson, R. A. et al. Global marine yield halved as fishing intensity redoubles. Fish Fish. http://dx.doi.org/10.1111/j.1467-2979.2012.00483.x (2012)
Acknowledgements
W.W.L.C. acknowledges funding support from the National Geographic Society and the Natural Sciences and Engineering Research Council of Canada. R.W. and D.P. were supported by the Pew Charitable Trust through the Sea Around Us project. We are grateful to S. Pauly and D. Palomares for reviewing the manuscript and providing the Aquamap distributions from FishBase, respectively.
Author information
Authors and Affiliations
Contributions
W.W.L.C. and D.P. designed the study. W.W.L.C. conducted the analysis. R.W. and D.P. provided the fisheries catch and effort data from the Sea Around Us project. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Tables 1-5, Supplementary Figures 1-3 and additional references. (PDF 3857 kb)
Rights and permissions
About this article
Cite this article
Cheung, W., Watson, R. & Pauly, D. Signature of ocean warming in global fisheries catch. Nature 497, 365–368 (2013). https://doi.org/10.1038/nature12156
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12156
This article is cited by
-
Natural warming differentiates communities and increases diversity in deep-sea Ridge Flank Hydrothermal Systems
Communications Biology (2024)
-
Underwater hyperspectral imaging technology has potential to differentiate and monitor scallop populations
Reviews in Fish Biology and Fisheries (2024)
-
Patterns of occurrence of the sub-Antarctic fur seal Arctocephalus tropicalis (Gray 1872) in Southern Brazil: climatic and environmental associations
Polar Biology (2024)
-
Climate change exacerbates nutrient disparities from seafood
Nature Climate Change (2023)
-
Reorganization of seagrass communities in a changing climate
Nature Plants (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.