Abstract
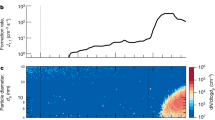
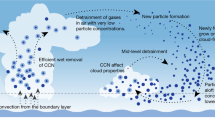
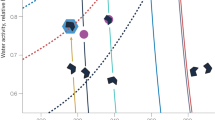
Atmospheric aerosols exert an important influence on climate1 through their effects on stratiform cloud albedo and lifetime2 and the invigoration of convective storms3. Model calculations suggest that almost half of the global cloud condensation nuclei in the atmospheric boundary layer may originate from the nucleation of aerosols from trace condensable vapours4, although the sensitivity of the number of cloud condensation nuclei to changes of nucleation rate may be small5,6. Despite extensive research, fundamental questions remain about the nucleation rate of sulphuric acid particles and the mechanisms responsible, including the roles of galactic cosmic rays and other chemical species such as ammonia7. Here we present the first results from the CLOUD experiment at CERN. We find that atmospherically relevant ammonia mixing ratios of 100 parts per trillion by volume, or less, increase the nucleation rate of sulphuric acid particles more than 100–1,000-fold. Time-resolved molecular measurements reveal that nucleation proceeds by a base-stabilization mechanism involving the stepwise accretion of ammonia molecules. Ions increase the nucleation rate by an additional factor of between two and more than ten at ground-level galactic-cosmic-ray intensities, provided that the nucleation rate lies below the limiting ion-pair production rate. We find that ion-induced binary nucleation of H2SO4–H2O can occur in the mid-troposphere but is negligible in the boundary layer. However, even with the large enhancements in rate due to ammonia and ions, atmospheric concentrations of ammonia and sulphuric acid are insufficient to account for observed boundary-layer nucleation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
IPCC. Climate Change 2007: the Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge Univ. Press, 2007)
Feingold, G. & Siebert, H. in Clouds in the Perturbed Climate System (eds Heintzenberg, J. & Charlson, R.J. ) 319–338 (MIT Press, 2009)
Rosenfeld, D. et al. Flood or drought: how do aerosols affect precipitation? Science 321, 1309–1313 (2008)
Merikanto, J., Spracklen, D. V., Mann, G. W., Pickering, S. J. & Carslaw, K. S. Impact of nucleation on global CCN. Atmos. Chem. Phys. 9, 8601–8616 (2009)
Spracklen, D. V. et al. Contribution of particle formation to global cloud condensation nuclei concentrations. Geophys. Res. Lett. 35, L06808 10.1029/2007GL033038 (2008)
Pierce, J. R. & Adams, P. J. Can cosmic rays affect cloud condensation nuclei by altering new particle formation rates? Geophys. Res. Lett. 36, L09820 10.1029/2009GL037946 (2009)
Zhang, R. Getting to the critical nucleus of aerosol formation. Science 328, 1366–1367 (2010)
Kerminen, V.-M. et al. Atmospheric nucleation: highlights of the EUCAARI project and future directions. Atmos. Chem. Phys. 10, 10829–10848 (2010)
Boy, M. et al. Sulphuric acid closure and contribution to nucleation mode particle growth. Atmos. Chem. Phys. 5, 863–878 (2005)
Wang, L. et al. Atmospheric nanoparticles formed from heterogeneous reactions of organics. Nature Geosci. 3, 238–242 (2010)
Ziereis, H. & Arnold, F. Gaseous ammonia and ammonium ions in the free troposphere. Nature 321, 503–505 (1986)
Coffman, D. J. & Hegg, D. A. A preliminary study of the effect of ammonia on particle nucleation in the marine boundary layer. J. Geophys. Res. 100 (D4), 7147–7160 (1995)
Ball, S. M., Hanson, D. R., Eisele, F. L. & McMurry, P. H. Laboratory studies of particle nucleation: initial results for H2SO4, H2O, and NH3 vapors. J. Geophys. Res. 104, 23709–23718 (1999)
Zhang, R. et al. Atmospheric new particle formation enhanced by organic acids. Science 304, 1487–1490 (2004)
Murphy, S. M. et al. Secondary aerosol formation from atmospheric reactions of aliphatic amines. Atmos. Chem. Phys. 7, 2313–2337 (2007)
Berndt, T. et al. Laboratory study on new particle formation from the reaction OH + SO2: influence of experimental conditions, H2O vapour, NH3 and the amine tert-butylamine on the overall process. Atmos. Chem. Phys. 10, 7101–7116 (2010)
Smith, J. N. et al. Observations of aminium salts in atmospheric nanoparticles and possible climatic implications. Proc. Natl Acad. Sci. USA 107, 6634–6639 (2010)
Zhang, R. et al. Formation of nanoparticles of blue haze enhanced by anthropogenic pollution. Proc. Natl Acad. Sci. USA 106, 17650–17654 (2009)
Metzger, A. et al. Evidence for the role of organics in aerosol particle formation under atmospheric conditions. Proc. Natl Acad. Sci. USA 107, 6646–6651 (2010)
Arnold, F. Multi-ion complexes in the stratosphere—implications for trace gases and aerosol. Nature 284, 610–611 (1980)
Raes, F., Janssens, A. & Van Dingenen, R. The role of ion-induced aerosol formation in the lower atmosphere. J. Aerosol Sci. 17, 466–470 (1986)
Turco, R. P., Zhao, J.-X. & Yu, F. A new source of tropospheric aerosols: Ion–ion recombination. Geophys. Res. Lett. 25, 635–638 (1998)
Lovejoy, E. R., Curtius, J. & Froyd, K. D. Atmospheric ion-induced nucleation of sulfuric acid and water. J. Geophys. Res. 109, D08204 10.1029/2003JD004460 (2004)
Sorokin, A. & Arnold, F. Laboratory study of cluster ions formation in H2SO4–H2O system: implications for threshold concentration of gaseous H2SO4 and ion-induced nucleation kinetics. Atmos. Environ. 41, 3740–3747 (2007)
Eichkorn, S., Wilhelm, S., Aufmhoff, H., Wohlfrom, K. H. & Arnold, F. Cosmic ray-induced aerosol-formation: first observational evidence from aircraft-based ion mass spectrometer measurements in the upper troposphere. Geophys. Res. Lett. 29, 1698–1701 (2002)
Lee, S. H. et al. Particle formation by ion nucleation in the upper troposphere and lower stratosphere. Science 301, 1886–1889 (2003)
Svensmark, H. & Friis-Christensen, E. Variation of cosmic ray flux and global cloud coverage—a missing link in solar-climate relationships. J. Atmos. Sol. Terr. Phys. 59, 1225–1232 (1997)
Kirkby, J. Cosmic rays and climate. Surv. Geophys. 28, 333–375 (2007)
Sipilä, M. et al. The role of sulfuric acid in atmospheric nucleation. Science 327, 1243–1246 (2010)
Kazil, J. & Lovejoy, E. R. A semi-analytical method for calculating rates of new sulfate aerosol formation from the gas phase. Atmos. Chem. Phys. 7, 3447–3459 (2007)
Kurtén, T., Loukonen, V., Vehkamäki, H. & Kulmala, M. Amines are likely to enhance neutral and ion-induced sulfuric acid–water nucleation in the atmosphere more effectively than ammonia. Atmos. Chem. Phys. 8, 4095–4103 (2008)
Kuang, C., McMurry, P. H., McCormick, A. V. & Eisele, F. L. Dependence of nucleation rates on sulfuric acid vapor concentration in diverse atmospheric locations. J. Geophys. Res. 113, D10209 0.1029/2007JD009253 (2008)
Paasonen, P. et al. Connection between new particle formation and sulfuric acid at Hohenpeissenberg (Germany) including the influence of organic compounds. Boreal Envir. Res. 14, 616–629 (2009)
Duplissy, J. et al. Results from the CERN pilot CLOUD experiment. Atmos. Chem. Phys. 10, 1635–1647 (2010)
Junninen, H. et al. A high-resolution mass spectrometer to measure atmospheric ion composition. Atmos. Meas. Tech. 3, 1039–1053 (2010)
Iida, K., Stolzenburg, M. R. & McMurry, P. H. Effect of working fluid on sub-2 nm particle detection with a laminar flow ultrafine condensation particle counter. Aerosol Sci. Technol. 43, 81–96 (2009)
Vanhanen, J. et al. Particle size magnifier for nano-CN detection. Aerosol Sci. Technol. 45, 533–542 (2011)
Brunelli, N. A., Flagan, R. C. & Giapis, K. P. Radial differential mobility analyzer for one nanometer particle classification. Aerosol Sci. Technol. 43, 53–59 (2009)
Kulmala, M. et al. Towards direct measurement of atmospheric nucleation. Science 318, 89–92 (2007)
Graus, M., Müller, M. & Hansel, A. High resolution PTR-TOF: quantification and formula confirmation of VOC in real time. J. Am. Soc. Mass Spectrom. 21, 1037–1044 (2010)
Norman, M., Hansel, A. & Wisthaler, A. O2 + as reagent ion in the PTR-MS instrument: detection of gas-phase ammonia. Int. J. Mass Spectrom. 265, 382–387 (2007)
Froyd, K. D. & Lovejoy, E. R. Experimental thermodynamics of cluster ions composed of H2SO4 and H2O. 2. Measurements and ab initio structures of negative ions. J. Phys. Chem. A 107, 9812–9824 (2003)
Kerminen, V.-M. & Kulmala, M. Analytical formulae connecting the ‘real’ and the ‘apparent’ nucleation rate and the nuclei number concentration for atmospheric nucleation events. J. Aerosol Sci. 33, 609–622 (2002)
Kulmala, M. & Kerminen, V.-M. On the formation and growth of atmospheric nanoparticles. Atmos. Res. 90, 132–150 (2008)
Acknowledgements
We thank CERN for supporting CLOUD with important technical and financial resources, and for providing a particle beam from the CERN Proton Synchrotron. We also thank J.-L. Agostini, S. Atieh, J. Baechler, D. Bloess, G. Bowden, A. Braem, T. Callamand, A. Castel, L.-P. De Menezes, G. Favre, L. Ferreira, L. Gatignon, D. Gregorio, M. Guinchard, E. Ivanova, F. Josa, I. Krasin, R. Kristic, A. Kuzmin, O. Maksumov, S. Mizin, R. Richter, R. Sitals, A. Vacca, R. Veenhof, A. Wasem and M. Wilhelmsson for their contributions to the experiment. This research has received funding from the EC Seventh Framework Programme under grant agreement no. 215072 (Marie Curie Initial Training Network, ‘CLOUD-ITN’) and ERC-Advanced Grant ‘ATMNUCLE’ no. 227463, the German Federal Ministry of Education and Research (project no. 01LK0902A), the Swiss National Science Foundation (project nos 206621_125025 and 206620_130527), the Academy of Finland Center of Excellence program (project no. 1118615), the Austrian Science Fund (FWF; project nos P19546 and L593), and the Russian Academy of Sciences and Russian Foundation for Basic Research (grant N08-02-91006-CERN).
Author information
Authors and Affiliations
Contributions
J.A. performed the nucleation rate analysis. S.S. conducted the APi-TOF analysis. J.A., F.B., M.B., A. Downard, E.D., J. Duplissy, S.E., A.F., S.G., D.H., L.I., W.J., J.K., F.K., A. Kürten, A. Kupc, K.L., V.M., A.M., T.N., F.R., L.R., R.S., S.S., Y.S., G.T. and D.W. conducted the data collection and analysis. J.A., K.S.C., J.C., E.D., S.E., L.I., E.R.L. and F.S. performed the modelling. J.K. wrote the manuscript. U.B., K.S.C., J.C., J.K., M.K., J.H.S. and D.R.W. did data interpretation and editing of the manuscript. All authors contributed to the development of the CLOUD facility and analysis instruments, and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Text and Data, Supplementary References and Supplementary Figures 1-4 with legends. (PDF 368 kb)
Rights and permissions
About this article
Cite this article
Kirkby, J., Curtius, J., Almeida, J. et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature 476, 429–433 (2011). https://doi.org/10.1038/nature10343
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10343
This article is cited by
-
Current and future machine learning approaches for modeling atmospheric cluster formation
Nature Computational Science (2023)
-
Increasing net ecosystem carbon budget and mitigating global warming potential with improved irrigation and nitrogen fertilization management of a spring wheat farmland system in arid Northwest China
Plant and Soil (2023)
-
Aerosols, Clusters, Greenhouse Gases, Trace Gases and Boundary-Layer Dynamics: on Feedbacks and Interactions
Boundary-Layer Meteorology (2023)
-
NO at low concentration can enhance the formation of highly oxygenated biogenic molecules in the atmosphere
Nature Communications (2023)
-
The gas-phase formation mechanism of iodic acid as an atmospheric aerosol source
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.