Abstract
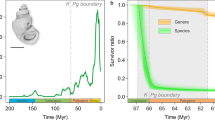
Palaeontologists characterize mass extinctions as times when the Earth loses more than three-quarters of its species in a geologically short interval, as has happened only five times in the past 540 million years or so. Biologists now suggest that a sixth mass extinction may be under way, given the known species losses over the past few centuries and millennia. Here we review how differences between fossil and modern data and the addition of recently available palaeontological information influence our understanding of the current extinction crisis. Our results confirm that current extinction rates are higher than would be expected from the fossil record, highlighting the need for effective conservation measures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Novacek, M. J. The Biodiversity Crisis: Losing What Counts (The New Press, 2001)
Jablonski, D. Extinctions in the fossil record. Phil. Trans. R. Soc. Lond. B 344, 11–17 (1994)This paper summarizes, from a palaeontological perspective, the difficulties of comparing the past and present extinctions.
Raup, D. M. & Sepkoski, J. J. Mass extinctions in the marine fossil record. Science 215, 1501–1503 (1982)This is a statistical assessment of the Big Five extinction rates relative to background rates.
Bambach, R. K. Phanerozoic biodiversity mass extinctions. Annu. Rev. Earth Planet. Sci. 34, 127–155 (2006)This paper discusses the definition of mass extinctions and mass depletions, and the relative role of origination versus extinction rates in causing the diversity reductions that characterize the Big Five.
Alroy, J. Dynamics of origination and extinction in the marine fossil record. Proc. Natl Acad. Sci. USA 105, 11536–11542 (2008)
IUCN. International Union for Conservation of Nature Red List 〈http://www.iucn.org/about/work/programmes/species/red_list/〉 (2010)
Pereira, H. M. et al. Scenarios for global biodiversity in the 21st century. Science 330, 1496–1501 (2010)
Ceballos, G. & Ehrlich, P. R. Mammal population losses and the extinction crisis. Science 296, 904–907 (2002)
Hughes, J. B., Daily, G. C. & Ehrlich, P. R. Population diversity: its extent and extinction. Science 278, 689–692 (1997)
Dirzo, R. & Raven, P. H. Global state of biodiversity and loss. Annu. Rev. Environ. Resour. 28, 137–167 (2003)This paper is an overview of the taxonomic and spatiotemporal patterns of biodiversity and the magnitude of the current biodiversity crisis.
Joppa, L. N., Roberts, D. L. & Pimm, S. L. How many species of flowering plants are there? Proc. R. Soc. Lond. B 10.1098/rspb.2010.1004 (2010)
Leakey, R. & Lewin, R. The Sixth Extinction: Patterns of Life and the Future of Humankind (Doubleday, 1992)
Wake, D. B. & Vredenburg, V. T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl Acad. Sci. USA 105, 11466–11473 (2008)
May, R. M., Lawton, J. H. & Stork, N. E. in Extinction Rates (eds Lawton, J. H. & May, R. M. ) Ch. 1, 1–24 (Oxford University Press, 1995)This paper compares fossil-background and recent extinction rates and explains the numerous assumptions that are required for the comparison.
Pimm, S. L., Russell, G. J., Gittleman, J. L. & Brooks, T. M. The future of biodiversity. Science 269, 347–350 (1995)This paper explains and uses the E/MSY metric to compare the fossil-background, current, and predicted future extinction rates.
Myers, N. Mass extinctions: what can the past tell us about the present and future? Palaeogeogr. Palaeoclimatol. Palaeoecol. 82, 175–185 (1990)
Pimm, S. L. & Brooks, T. M. in Nature and Human Society: The Quest for a Sustainable World (eds Raven, P. H. & Williams, T. ) 46–62 (National Academy Press, 1997)
Barnosky, A. D. Heatstroke: Nature in an Age of Global Warming 1–269 (Island Press, 2009)
Vredenburg, V. T., Knapp, R. A., Tunstall, T. S. & Briggs, C. J. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl Acad. Sci. USA 107, 9689–9694 (2010)
Hoffmann, M. et al. The impact of conservation on the status of the world’s vertebrates. Science 330, 1503–1509 (2010)
Avise, J. C., Walker, D. & Johns, G. C. Speciation durations and Pleistocene effects on vertebrate phylogeography. Proc. R. Soc. Lond. B 265, 1707–1712 (1998)
Weir, J. T. & Schluter, D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 315, 1574–1576 (2007)
Lu, P. J., Yogo, M. & Marshall, C. R. Phanerozoic marine biodiversity dynamics in light of the incompleteness of the fossil record. Proc. Natl Acad. Sci. USA 103, 2736–2739 (2006)
Vié, J.-C., Hilton-Taylor, C. & Stuart, S. N. (eds) Wildlife in a Changing World—An Analysis of the 2008 IUCN Red List of Threatened Species 180 (IUCN, 2009)
Baillie, J. E. M. et al. Toward monitoring global biodiversity. Conserv. Lett. 1, 18–26 (2008)
Şengör, A. M. C., Atayman, S. & Özeren, S. A scale of greatness and causal classification of mass extinctions: implications for mechanisms. Proc. Natl Acad. Sci. USA 105, 13736–13740 (2008)
Pimm, S., Raven, P., Peterson, A., Sekercioglu, Ç. H. & Ehrlich, P. R. Human impacts on the rates of recent, present, and future bird extinctions. Proc. Natl Acad. Sci. USA 103, 10941–10946 (2006)
Foote, M. Temporal variation in extinction risk and temporal scaling of extinction metrics. Paleobiology 20, 424–444 (1994)This paper addresses the effect of interval length on extinction metrics using simulations.
Foote, M. & Raup, D. M. Fossil preservation and the stratigraphic ranges of taxa. Paleobiology 22, 121–140 (1996)
PBDB. The Paleobiology Database 〈http://paleodb.org/cgi-bin/bridge.pl〉 (2010)
NEOMAP. The Neogene Mammal Mapping Portal 〈http://www.ucmp.berkeley.edu/neomap/〉 (2010)
Barnosky, A. D. Megafauna biomass tradeoff as a driver of Quaternary and future extinctions. Proc. Natl Acad. Sci. USA 105, 11543–11548 (2008)
Koch, P. L. & Barnosky, A. D. Late Quaternary extinctions: state of the debate. Annu. Rev. Ecol. Evol. Syst. 37, 215–250 (2006)
Mace, G. M. et al. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv. Biol. 22, 1424–1442 (2008)This paper explains the methodology used by the IUCN to assess the extinction risks of extant species.
Alroy, J. Constant extinction, constrained diversification, and uncoordinated stasis in North American mammals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 127, 285–311 (1996)
Stork, N. E. Re-assessing current extinction rates. Biodivers. Conserv. 19, 357–371 (2010)
Jablonski, D. Lessons from the past: evolutionary impacts of mass extinctions. Proc. Natl Acad. Sci. USA 98, 5393–5398 (2001)
Jablonski, D. Extinction and the spatial dynamics of biodiversity. Proc. Natl Acad. Sci. USA 105, 11528–11535 (2008)
Purvis, A., Jones, K. E. & Mace, G. M. Extinction. Bioessays 22, 1123–1133 (2000)
Agapow, P.-M. et al. The impact of the species concept on biodiversity studies. Q. Rev. Biol. 79, 161–179 (2004)
Alroy, J. et al. Phanerozoic trends in the global diversity of marine invertebrates. Science 321, 97–100 (2008)
Steadman, D. W. Extinction and Biogeography of Tropical Pacific Birds (University of Chicago Press, 2006)
Raup, D. M. & Jablonski, D. Geography of end-Cretaceous marine bivalve extinctions. Science 260, 971–973 (1993)
Regan, H. M., Lupia, R., Drinnan, A. N. & Burgman, M. A. The currency and tempo of extinction. Am. Nat. 157, 1–10 (2001)
Carrasco, M. A., Barnosky, A. D. & Graham, R. W. Quantifying the extent of North American mammal extinction relative to the pre-anthropogenic baseline. PLoS ONE 4, e8331 (2009)This paper uses species-area relationships based on fossil data to demonstrate that the recent biodiversity baseline for mammals is substantially depressed with respect to its normal condition.
Quental, T. B. & Marshall, C. R. Diversity dynamics: molecular phylogenies need the fossil record. Trends Ecol. Evol. 25, 434–441 (2010)
Anderson, C. N. K., Ramakrishnan, U., Chan, Y. L. & Hadly, E. A. Serial SimCoal: a population genetics model for data from multiple populations and points in time. Bioinformatics 21, 1733–1734 (2005)
Ramakrishnan, U. & Hadly, E. A. Using phylochronology to reveal cryptic population histories: review and synthesis of four ancient DNA studies. Mol. Ecol. 18, 1310–1330 (2009)This paper uses a hypothesis-testing framework to reveal population histories and compare past populations to present ones.
Blois, J. L., McGuire, J. L. & Hadly, E. A. Small mammal diversity loss in response to late-Pleistocene climatic change. Nature 465, 771–774 (2010)
Cardillo, M. et al. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 (2005)
Davies, T. J. et al. Phylogenetic trees and the future of mammalian biodiversity. Proc. Natl Acad. Sci. USA 105, 11556–11563 (2008)
Isaac, N. J. B., Turvey, S. T., Collen, B., Waterman, C. & Baillie, J. E. M. Mammals on the EDGE: conservation priorities based on threat and phylogeny. PLoS ONE 2, e296 (2007)
Russell, G. J., Brooks, T. M., McKinney, M. M. & Anderson, C. G. Present and future taxonomic selectivity in bird and mammal extinctions. Conserv. Biol. 12, 1365–1376 (1998)
Erwin, D. H. The Permo-Triassic extinction. Nature 367, 231–236 (1994)
Arens, N. C. & West, I. D. Press-pulse: a general theory of mass extinction? Paleobiology 34, 456–471 (2008)
Brook, B. W., Sodhi, N. S. & Bradshaw, C. J. A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 (2008)
Jablonski, D. in Dynamics of Extinction (ed. Elliott, D. K. ) 183–229 (Wiley, 1986)
Alvarez, L. W., Alvarez, W., Asaro, F. & Michel, H. V. Extraterrestrial cause for the Cretaceous-Tertiary extinction. Science 208, 1095–1108 (1980)
Schulte, P. et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 327, 1214–1218 (2010)
Prauss, M. L. The K/Pg boundary at Brazos-River, Texas, USA—an approach by marine palynology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 283, 195–215 (2009)
GBO3. Global Biodiversity Outlook 3 94 (Secretariat of the Convention on Biological Diversity, 2010)
Mace, G. et al. in Millenium Ecosystem Assessment, Ecosystems and Human Well-being: Biodiversity Synthesis (eds Ceballos, S. L. G., Orians, G. & Pacala, S. ) Ch. 4, 77–122 (World Resources Institute, 2005)
Cardillo, M., Mace, G. M., Gittleman, J. L. & Purvis, A. Latent extinction risk and the future battlegrounds of mammal conservation. Proc. Natl Acad. Sci. USA 103, 4157–4161 (2006)
Sepkoski, J. J. in Global Events and Event Stratigraphy in the Phanerozoic (ed. Walliser, O. H. ) 35–51 (Springer, 1996)
Sheehan, P. M. The Late Ordovician mass extinction. Annu. Rev. Earth Planet. Sci. 29, 331–364 (2001)
Sutcliffe, O. E., Dowdeswell, J. A., Whittington, R. J., Theron, J. N. & Craig, J. Calibrating the Late Ordovician glaciation and mass extinction by the eccentricity cycles of Earth's orbit. Geology 28, 967–970 (2000)
Sandberg, C. A., Morrow, J. R. & Zlegler, W. in Catastrophic Events and Mass Extinctions: Impacts and Beyond (eds Koeberl, C. & MacLeod, K. G. ) 473–387 (Geological Society of America Special Paper 356, GSA, 2002)
McGhee, G. R. The Late Devonian Mass Extinction 1–302 (Columbia University Press, 1996)
Murphy, A. E., Sageman, B. B. & Hollander, D. J. Eutrophication by decoupling of the marine biogeochemical cycles of C, N, and P: a mechanism for the Late Devonian mass extinction. Geology 28, 427–430 (2000)
Algeo, T. J., Scheckler, S. E. & Maynard, J. B. in Plants Invade the Land: Evolutionary and Environmental Approaches (eds Gensel, P. G. & Edwards, D. ) 213–236 (Columbia University Press, 2000)
Berner, R. A. Examination of hypotheses for the Permo-Triassic boundary extinction by carbon cycle modeling. Proc. Natl Acad. Sci. USA 99, 4172–4177 (2002)
Payne, J. L. et al. Calcium isotope constraints on the end-Permian mass extinction. Proc. Natl Acad. Sci. USA 107, 8543–8548 (2010)
Knoll, A. H., Bambach, R. K., Payne, J. L., Pruss, S. & Fischer, W. W. Paleophysiology and end-Permian mass extinction. Earth Planet. Sci. Lett. 256, 295–313 (2007)
Hesselbo, S. P., McRoberts, C. A. & Palfy, J. Triassic-Jurassic boundary events: problems, progress, possibilities. Palaeogeogr. Palaeoclimatol. Palaeoecol. 244, 1–10 (2007)
Ward, P. D. et al. Sudden productivity collapse associated with the Triassic-Jurassic boundary mass extinction. Science 292, 1148–1151 (2001)
Archibald, J. D. et al. Cretaceous extinctions: multiple causes. Science 328, 973 (2010)
Keller, G. Cretaceous climate, volcanism, impacts, and biotic effects. Cretac. Res. 29, 754–771 (2008)
Mukhopadhyay, S., Farley, K. A. & Montanari, A. A short duration of the Cretaceous-Tertiary boundary event: evidence from extraterrestrial helium-3. Science 291, 1952–1955 (2010)
Royer, D. L. CO2-forced climate thresholds during the Phanerozoic. Geochim. Cosmochim. Acta 70, 5665–5675 (2006)
McCallum, M. L. Amphibian decline or extinction? Current declines dwarf background extinction rate. J. Herpetol. 41, 483–491 (2007)
Rosenzweig, M. L. Loss of speciation rate will impoverish future diversity. Proc. Natl Acad. Sci. USA 98, 5404–5410 (2001)
Thomas, C. D. et al. Extinction risk from climate change. Nature 427, 145–148 (2004)
Pimm, S. L. & Raven, P. H. Extinction by numbers. Nature 403, 843–845 (2000)
Sinervo, B. et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899 (2010)
Roelants, K. et al. Global patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA 104, 887–892 (2007)
Raup, D. M. A kill curve for Phanerozoic marine species. Paleobiology 17, 37–48 (1991)
Foote, M. Estimating taxonomic durations and preservation probability. Paleobiology 23, 278–300 (1997)
Rabosky, D. L. Extinction rates should not be estimated from molecular phylogenies. Evolution 64, 1816–1824 (2010)
Barnosky, A. D. & Lindsey, E. L. Timing of Quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quat. Int. 217, 10–29 (2010)
Turvey, S. T. Holocene Extinctions (Oxford University Press, 2009)
Faith, J. T. & Surovell, T. A. Synchronous extinction of North America’s Pleistocene mammals. Proc. Natl Acad. Sci. USA 106, 20641–20645 (2009)
Surovell, T., Waguespack, N. & Brantingham, P. J. Global archaeological evidence for proboscidean overkill. Proc. Natl Acad. Sci. USA 102, 6231–6236 (2005)
Finlayson, C. et al. Late survival of Neanderthals at the southernmost extreme of Europe. Nature 443, 850–853 (2006)
Morwood, M. J. et al. Archaeology and age of a new hominin from Flores in eastern Indonesia. Nature 431, 1087–1091 (2004)
Orlova, L. A., Vasil’ev, S. K., Kuz’min, Y. V. & Kosintsev, P. A. New data on the time and place of extinction of the woolly rhinoceros Coelodonta antiquitatis Blumenbach, 1799. Dokl. Akad. Nauk 423, 133–135 (2008)
Reumer, J. W. F. et al. Late Pleistocene survival of the saber-toothed cat Homotherium in Northwestern Europe. J. Vertebr. Paleontol. 23, 260–262 (2003)
MacPhee, R. D. E. Extinctions in Near Time: Causes, Contexts, and Consequences (Kluwer Academic/Plenum Publishers, 1999)
Acknowledgements
S. Beissinger, P. Ehrlich, E. Hadly, A. Hubbe, D. Jablonski, S. Pimm and D. Wake provided constructive comments. Paleobiology Database data were contributed by M. Carrano, J. Alroy, M. Uhen, R. Butler, J. Mueller, L. van den Hoek Ostende, J. Head, E. Fara, D. Croft, W. Clyde, K. Behrensmeyer, J. Hunter, R. Whatley and W. Kiessling. The work was funded in part by NSF grants EAR-0720387 (to A.D.B.) and DEB-0919451 (supporting N.M.). This is University of California Museum of Paleontology Contribution 2024.
Author information
Authors and Affiliations
Contributions
All authors participated in literature review and contributed to discussions that resulted in this paper. A.D.B. planned the project, analysed and interpreted data, and wrote the paper. N.M. and S.T. performed key data analyses and interpretation relating to rate comparisons. G.O.U.W., B.S. and E.L.L. assembled critical data. T.B.Q. and C.M. provided data, analyses and ideas relating to diversity dynamics and rate-magnitude comparisons. J.L.M. helped produce figures and with N.M., S.T., G.O.U.W., B.S., T.B.Q., C.M., K.C.M., B.M. and E.A.F. contributed to finalizing the text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Barnosky, A., Matzke, N., Tomiya, S. et al. Has the Earth’s sixth mass extinction already arrived?. Nature 471, 51–57 (2011). https://doi.org/10.1038/nature09678
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09678
This article is cited by
-
Weather explains the decline and rise of insect biomass over 34 years
Nature (2024)
-
Loss of amphibian species alters periphyton communities in montane ponds
Hydrobiologia (2024)
-
Assessing the applicability of binary land-cover variables to species distribution models across multiple grains
Landscape Ecology (2024)
-
A Naturalistic Theodicy for Sterba’s Problem of Natural Evil
Sophia (2024)
-
The effects of salinity on the distribution and survival of two exotic ostracods in the Iberian Peninsula
Hydrobiologia (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.