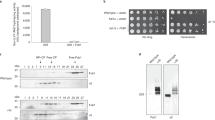
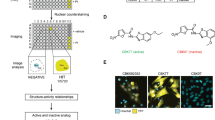
Abstract
Proteasomes, the primary mediators of ubiquitin–protein conjugate degradation, are regulated through complex and poorly understood mechanisms. Here we show that USP14, a proteasome-associated deubiquitinating enzyme, can inhibit the degradation of ubiquitin–protein conjugates both in vitro and in cells. A catalytically inactive variant of USP14 has reduced inhibitory activity, indicating that inhibition is mediated by trimming of the ubiquitin chain on the substrate. A high-throughput screen identified a selective small-molecule inhibitor of the deubiquitinating activity of human USP14. Treatment of cultured cells with this compound enhanced degradation of several proteasome substrates that have been implicated in neurodegenerative disease. USP14 inhibition accelerated the degradation of oxidized proteins and enhanced resistance to oxidative stress. Enhancement of proteasome activity through inhibition of USP14 may offer a strategy to reduce the levels of aberrant proteins in cells under proteotoxic stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Finley, D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78, 477–513 (2009)
Schrader, E. K., Harstad, K. G. & Matouschek, A. Targeting proteins for degradation. Nature Chem. Biol. 5, 815–822 (2009)
Thrower, J. S., Hoffman, L., Rechsteiner, M. & Pickart, C. M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 19, 94–102 (2000)
Verma, R. et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 298, 611–615 (2002)
Yao, T. & Cohen, R. E. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature 419, 403–407 (2002)
Lam, Y. A., Xu, W., DeMartino, G. N. & Cohen, R. E. Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature 385, 737–740 (1997)
Koulich, E., Li, X. & DeMartino, G. N. Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol. Biol. Cell 19, 1072–1082 (2008)
Jacobson, A. D. et al. The lysine 48 and lysine 63 ubiquitin conjugates are processed differently by the 26S proteasome. J. Biol. Chem. 284, 35485–35494 (2009)
Verma, R. et al. Proteasomal proteomics: identification of nucleotide-sensitive proteasome-interacting proteins by mass spectrometric analysis of affinity-purified proteasomes. Mol. Biol. Cell 11, 3425–3439 (2000)
Borodovsky, A. et al. A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J. 20, 5187–5196 (2001)
Leggett, D. S. et al. Multiple associated proteins regulate proteasome structure and function. Mol. Cell 10, 495–507 (2002)
Wilson, S. M. et al. Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nature Genet. 32, 420–425 (2002)
Chernova, T. A. et al. Pleiotropic effects of Ubp6 loss on drug sensitivities and yeast prion are due to depletion of the free ubiquitin pool. J. Biol. Chem. 278, 52102–52115 (2003)
Anderson, C. et al. Loss of Usp14 results in reduced levels of ubiquitin in ataxia mice. J. Neurochem. 95, 724–731 (2005)
Hu, M. et al. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme Usp14. EMBO J. 24, 3747–3756 (2005)
Hanna, J. et al. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell 127, 99–111 (2006)
Hanna, J., Meides, A., Zhang, D. P. & Finley, D. A ubiquitin stress response induces altered proteasome composition. Cell 129, 747–759 (2007)
Crimmins, S. et al. Transgenic rescue of ataxia mice with neuronal-specific expression of ubiquitin-specific protease 14. J. Neurosci. 26, 11423–11431 (2006)
Crimmins, S. et al. Transgenic rescue of ataxia mice reveals a male-specific sterility defect. Dev. Biol. 325, 33–42 (2009)
Chen, P.-C. et al. The proteasome-associated deubiquitinating enzyme Usp14 is essential for the maintenance of synaptic ubiquitin levels and the development of neuromuscular junctions. J. Neurosci. 29, 10909–10919 (2009)
Peth, A., Besche, H. C. & Goldberg, A. L. Ubiquitinated proteins activate the proteasome by binding to Usp14/Upb6, which cause 20S gate opening. Mol. Cell 36, 794–804 (2009)
Catic, A. et al. Screen for ISG15-crossreactive deubiquitinases. PLoS ONE 2, e679 (2007)
Wang, X. et al. Mass spectrometric characterization of the affinity-purified human 26S proteasome complex. Biochemistry 46, 3553–3565 (2007)
Yao, T. et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nature Cell Biol. 8, 994–1002 (2006)
Spires-Jones, T. L., Stoothoff, W. H., de Calignon, A., Jones, P. B. & Hyman, B. T. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 32, 150–159 (2009)
Kwong, L. K., Uryu, K., Trojanowski, J. Q. & Lee, V. M. TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis. Neurosignals 16, 41–51 (2008)
David, D. C. et al. Proteasomal degradation of tau protein. J. Neurochem. 83, 176–185 (2002)
Petrucelli, L. et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 13, 703–714 (2004)
Todi, S. V. et al. Cellular turnover of the polyglutamine disease protein ataxin-3 is regulated by its catalytic activity. J. Biol. Chem. 282, 29348–29358 (2007)
Varshavsky, A., Turner, G., Du, F. & Xie, Y. The ubiquitin system and the N-end rule pathway. Biol. Chem. 381, 779–789 (2000)
Dantuma, N. P., Lindsten, K., Glas, R., Jellne, M. & Masucci, M. G. Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nature Biotechnol. 18, 538–543 (2000)
Saeki, Y., Isono, E. & Toh-E, A. Preparation of ubiquitinated substrates by the PY motif-insertion method for monitoring proteasome activity. Methods Enzymol. 399, 215–227 (2005)
Kirkpatrick, D. S. et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nature Cell Biol. 8, 700–710 (2006)
Amerik, A. Y., Li, S. J. & Hochstrasser, M. Analysis of the deubiquitinating enzymes of the yeast Saccharomyces cerevisiae . Biol. Chem. 381, 981–992 (2000)
Hanna, J., Leggett, D. S. & Finley, D. Ubiquitin depletion as a key mediator of toxicity by translational inhibitors. Mol. Cell. Biol. 23, 9251–9261 (2003)
Shabek, N., Herman-Bachinsky, Y. & Ciechanover, A. Ubiquitin degradation with its substrate, or as a monomer in a ubiquitination-independent mode, provides clues to proteasome regulation. Proc. Natl Acad. Sci. USA 106, 11907–11912 (2009)
Hoyt, M. A., Zhang, M. & Coffino, P. Probing the ubiquitin/proteasome system with ornithine decarboxylase, a ubiquitin-independent substrate. Methods Enzymol. 398, 399–413 (2005)
Stadtman, E. R. Protein oxidation and aging. Free Radic. Res. 40, 1250–1258 (2006)
Ahmed, E. K., Picot, C. R., Bulteau, A. L. & Friguet, B. Protein oxidative modifications and replicative senescence of WI-38 human embryonic fibroblasts. Ann. NY Acad. Sci. 1119, 88–96 (2007)
Hamazaki, J. et al. A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO J. 25, 4524–4536 (2006)
Qiu, X. B. et al. hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO J. 25, 5742–5753 (2006)
Husnjak, K. et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 453, 481–488 (2008)
Chauhan, D., Bianchi, G. & Anderson, K. C. Targeting the UPS as therapy in multiple myeloma. BMC Biochem. 9 (Suppl. 1). S1 (2008)
Muchamuel, T. et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nature Med. 15, 781–787 (2009)
Chondrogianni, N. et al. Overexpression of proteasome β5 subunit increases the amount of assembled proteasome and confers ameliorated response to oxidative stress and higher survival rates. J. Biol. Chem. 280, 11840–11850 (2005)
Tonoki, A. et al. Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol. Cell. Biol. 29, 1095–1106 (2009)
Lehman, N. L. The ubiquitin proteasome system in neuropathology. Acta Neuropathol. 118, 329–347 (2009)
Hinault, M. P., Ben-Zvi, A. & Goloubinoff, P. Chaperones and proteases: cellular fold-controlling factors of proteins in neurodegenerative diseases and aging. J. Mol. Neurosci. 30, 249–265 (2006)
Balch, W. E., Morimoto, R. I., Dillin, A. & Kelly, J. W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008)
Goldberg, A. L. Protein degradation and protection against misfolded and damaged proteins. Nature 426, 895–899 (2003)
Sowa, M. E., Bennett, E. J., Gygi, S. P. & Harper, J. W. Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 (2009)
Elsasser, S., Schmidt, M. & Finley, D. Characterization of the proteasome using native gel electrophoresis. Methods Enzymol. 398, 353–363 (2005)
Kleijnen, M. F. et al. Stability of the proteasome can be regulated allosterically through engagement of its proteolytic active sites. Nature Struct. Mol. Biol. 14, 1180–1188 (2007)
Kusmierczyk, A. R., Kunjappu, M. J., Funakoshi, M. & Hochstrasser, M. A multimeric assembly factor controls the formation of alternative 20S proteasomes. Nature Struct. Mol. Biol. 15, 237–244 (2008)
Park, S. et al. Hexameric assembly of the proteasomal ATPases is templated through their C termini. Nature 459, 866–870 (2009)
Malo, N., Hanley, J., Cerquozzi, S., Pelletier, J. & Nadon, R. Statistical practice in high-throughput screening data analysis. Nature Biotechnol. 24, 167–175 (2006)
Kobayashi, H. et al. Hrs, a mammalian master molecule in vesicular transport and protein sorting, suppresses the degradation of ESCRT proteins signal transducing adaptor molecule 1 and 2. J. Biol. Chem. 280, 10468–10477 (2005)
Kuma, A. et al. The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 (2004)
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010)
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 (1983)
Li, X., Traganos, F., Melamed, M. R. & Darzynkiewicz, Z. Single-step procedure for labeling DNA strand breaks with fluorescein- or BODIPY-conjugated deoxynucleotides: detection of apoptosis and bromodeoxyuridine incorporation. Cytometry 20, 172–180 (1995)
Jordan, M. A., Thrower, D. & Wilson, L. Mechanism of inhibition of cell proliferation by Vinca Alkaloids. Cancer Res. 51, 2212–2222 (1991)
Chondrogianni, N. et al. Central role of the proteasome in senescence and survival of human fibroblasts. J. Biol. Chem. 278, 28026–28037 (2003)
Kwak, M. K. et al. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol. Cell. Biol. 23, 8786–8794 (2003)
Acknowledgements
We thank K. Gordon, J. Y. Suk and N. Bays for advice and assistance, and members of the Finley laboratory for comments on the manuscript. We thank C. Shamu and the staff of the ICCB facility at Harvard Medical School, where the HT screen was carried out. We also thank N. Hathaway for ubiquitinated cyclin B, L. Huang for the tagged proteasome cell line, R. Baker for anti-USP14 antibody, G. DeMartino for anti-UCH37 antibody, K. Wilkinson and K. Walters for DUB enzymes, as well as C. Seong, M. Kim, S. M. Lim and D. Waterman for assistance in some experiments. For plasmids, we thank K. Walters, M. Sowa, W. Harper, V. Lee, F. Baralle, H. Paulson, Y. T. Kwon, M. Masucci, M.-K. Kwak, P. Coffino and C. Kahana. This work was supported by grants from the National Institutes of Health (DK082906 to D.F., GM65592 to D.F., GM66492 to R.W.K. and NS047533 to S.M.W.); the Harvard Technology Development Accelerator Fund (D.F.); Merck & Co. (D.F. and R.W.K.); and Johnson & Johnson (D.F. and R.W.K.).
Author information
Authors and Affiliations
Contributions
B.-H.L. carried out screening and most in vitro studies, and M.J.L. chemical analysis and most cell-based assays. R.W.K. and D.F. were responsible for overall design and oversight of the project. S.P., S.E. and N.D. provided skilled assistance in proteasome biochemistry and assays. D.-C.O., C.G. and S.P.G. designed and carried out chemistry studies. P.-C.C., S.M.W. and J.H. provided key reagents and intellectual input. Many authors contributed to preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
There is a patent application on this work, filed by Harvard University on behalf of the authors.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-29 with legends and additional references. (PDF 4052 kb)
Supplementary Table
This table contains a summary of nonspecific and weak hits. (PDF 907 kb)
Rights and permissions
About this article
Cite this article
Lee, BH., Lee, M., Park, S. et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467, 179–184 (2010). https://doi.org/10.1038/nature09299
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature09299
This article is cited by
-
Cardiac proteostasis in obesity and cardiovascular disease
Herz (2024)
-
Targeting ubiquitin specific proteases (USPs) in cancer immunotherapy: from basic research to preclinical application
Journal of Experimental & Clinical Cancer Research (2023)
-
A self-amplifying USP14-TAZ loop drives the progression and liver metastasis of pancreatic ductal adenocarcinoma
Cell Death & Differentiation (2023)
-
Discovery of an OTUD3 inhibitor for the treatment of non-small cell lung cancer
Cell Death & Disease (2023)
-
The Emerging Roles of E3 Ligases and DUBs in Neurodegenerative Diseases
Molecular Neurobiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.