Abstract
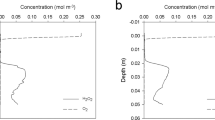
Some bacteria are capable of extracellular electron transfer, thereby enabling them to use electron acceptors and donors without direct cell contact1,2,3,4. Beyond the micrometre scale, however, no firm evidence has previously existed that spatially segregated biogeochemical processes can be coupled by electric currents in nature. Here we provide evidence that electric currents running through defaunated sediment couple oxygen consumption at the sediment surface to oxidation of hydrogen sulphide and organic carbon deep within the sediment. Altering the oxygen concentration in the sea water overlying the sediment resulted in a rapid (<1-h) change in the hydrogen sulphide concentration within the sediment more than 12 mm below the oxic zone, a change explicable by transmission of electrons but not by diffusion of molecules. Mass balances indicated that more than 40% of total oxygen consumption in the sediment was driven by electrons conducted from the anoxic zone. A distinct pH peak in the oxic zone could be explained by electrochemical oxygen reduction, but not by any conventional sets of aerobic sediment processes. We suggest that the electric current was conducted by bacterial nanowires combined with pyrite, soluble electron shuttles and outer-membrane cytochromes. Electrical communication between distant chemical and biological processes in nature adds a new dimension to our understanding of biogeochemistry and microbial ecology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Reguera, G. et al. Extracellular electron transfer via microbial nanowires. Nature 435, 1098–1101 (2005)
Lovley, D. R., Coates, J. D., Blunt-Harris, E. L., Phillips, E. J. P. & Woodward, J. C. Humic substances as electron acceptors for microbial respiration. Nature 382, 445–448 (1996)
Leang, C., Coppi, M. V. & Lovley, D. R. OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J. Bacteriol. 185, 2096–2103 (2003)
Rabaey, K. et al. Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J. 1, 9–18 (2007)
Reimers, C. E., Tender, L. M., Fertig, S. & Wang, W. Harvesting energy from the marine sediment-water interface. Environ. Sci. Technol. 35, 192–195 (2001)
Ryckelynck, N., Stecher, H. A. & Reimers, C. E. Understanding the anodic mechanism of a seafloor fuel cell: interactions between geochemistry and microbial activity. Biogeochemistry 76, 113–139 (2005)
Canfield, D. E. et al. Pathways of organic carbon oxidation in three continental margin sediments. Mar. Geol. 113, 27–40 (1993)
Sayama, M., Risgaard-Petersen, N., Nielsen, L. P., Fossing, H. & Christensen, P. B. Impact of bacterial NO3 - transport on sediment biogeochemistry. Appl. Environ. Microbiol. 71, 7575–7577 (2005)
Risgaard-Petersen, N. et al. Evidence for complete denitrification in a benthic foraminifer. Nature 443, 93–96 (2006)
Glud, R. N. & Fenchel, T. The importance of ciliates for interstitial solute transport in benthic communities. Mar. Ecol. Prog. Ser. 186, 87–93 (1999)
Glud, R. N., Forster, S. & Huettel, M. Influence of radial pressure gradients on solute exchange in stirred benthic chambers. Mar. Ecol. Prog. Ser. 141, 303–311 (1996)
Soetaert, K., Hofmann, A. F., Middelburg, J. J., Meysman, F. J. R. & Greenwood, J. The effect of biogeochemical processes on pH. Mar. Chem. 105, 30–51 (2007)
Crank, J. The Mathematics of Diffusion 1st edn, 18–19 (Clarendon, 1967)
Li, Y.-H. & Gregory, S. Diffusion of ions in sea water and in deep-sea sediments. Geochim. Cosmochim. Acta 38, 703–714 (1974)
Ullman, W. J. & Aller, R. C. Diffusion coefficients in nearshore marine sediments. Limnol. Oceanogr. 27, 552–556 (1982)
Sato, M. & Mooney, H. M. The electrochemical mechanism of sulfide self-potentials. Geophysics 25, 226–249 (1960)
Bigalke, J. & Grabner, E. W. The geobattery model: a contribution to large scale electrochemistry. Electrochim. Acta 42, 3443–3452 (1997)
Naudet, V. & Revil, A. A sandbox experiment to investigate bacteria-mediated redox processes on self-potential signals. Geophys. Res. Lett. 32, L11405 (2005)
Ntarlagiannis, D., Atekwana, E. A., Hill, E. A. & Gorby, Y. Microbial nanowires: is the subsurface “hardwired”? Geophys. Res. Lett. 34, L17305 (2007)
Corry, C. E. Spontaneous polarization associated with porphyry sulfide mineralization. Geophysics 50, 1020–1034 (1985)
Leney, G. W. On: “Spontaneous polarization associated with porphyry sulfide mineralization” by C. E. Corry (GEOPHYSICS, 50, 1020–1034, June 1985). Geophysics 51, 1153–1154 (1986)
Williams, K. H., Hubbard, S. S. & Banfield, J. F. Galvanic interpretation of self-potential signals associated with microbial sulfate-reduction. J. Geophys. Res. 112, G03019 (2007)
Torres, C. I., Marcus, A. K., Parameswaran, P. & Rittmann, B. E. Kinetic experiments for evaluating the Nernst-Monod model for anode-respiring bacteria (ARB) in a biofilm anode. Environ. Sci. Technol. 42, 6593–6597 (2008)
Glud, R. N. Oxygen dynamics of marine sediments. Mar. Biol. Res. 4, 243–289 (2008)
Jeroschewski, P., Steuckart, C. & Kuhl, M. An amperometric microsensor for the determination of H2S in aquatic environments. Anal. Chem. 68, 4351–4357 (1996)
Stumm, W. & Morgan, J. J. Aquatic Chemistry 2nd edn, 179–206 (Wiley, 1981)
Stookey, L. L. Ferrozine - a new spectrophotometric reagent for iron. Anal. Chem. 42, 779–781 (1970)
Fossing, H. & Jorgensen, B. B. Measurement of bacterial sulfate reduction in sediments - evaluation of a single-step chromium reduction method. Biogeochemistry 8, 205–222 (1989)
Tromp, T. K., VanCappellen, P. & Key, R. M. A global model for the early diagenesis of organic carbon and organic phosphorous in marine sediments. Geochim. Cosmochim. Acta 59, 1259–1284 (1995)
Berg, P., Risgaard-Petersen, N. & Rysgaard, S. Interpretation of measured concentration profiles in sediment pore water. Limnol. Oceanogr. 43, 1500–1510 (1998)
Acknowledgements
This research was financially supported by the Aarhus University Research Foundation, the Danish National Research Foundation (N.R.-P.), the Max Planck Society (N.R.-P.) and the Japan Society for the Promotion of Science (M.S.).
Author Contributions The study was conceived by L.P.N. All authors contributed to the design and execution of the study, interpretation of the results and preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nielsen, L., Risgaard-Petersen, N., Fossing, H. et al. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 463, 1071–1074 (2010). https://doi.org/10.1038/nature08790
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08790
This article is cited by
-
Lebende Stromkabel mit überraschender Arbeitsteilung
BIOspektrum (2024)
-
Genomic insight of sulfate reducing bacterial genus Desulfofaba reveals their metabolic versatility in biogeochemical cycling
BMC Genomics (2023)
-
Coupled iron cycling and organic matter transformation across redox interfaces
Nature Reviews Earth & Environment (2023)
-
Cable bacteria with electric connection to oxygen attract flocks of diverse bacteria
Nature Communications (2023)
-
Suitability of lectin binding studies for the characterization of redox-active microbial environmental biofilms
AMB Express (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.