Abstract
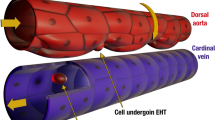
Biomechanical forces are emerging as critical regulators of embryogenesis, particularly in the developing cardiovascular system1,2. After initiation of the heartbeat in vertebrates, cells lining the ventral aspect of the dorsal aorta, the placental vessels, and the umbilical and vitelline arteries initiate expression of the transcription factor Runx1 (refs 3–5), a master regulator of haematopoiesis, and give rise to haematopoietic cells4. It remains unknown whether the biomechanical forces imposed on the vascular wall at this developmental stage act as a determinant of haematopoietic potential6. Here, using mouse embryonic stem cells differentiated in vitro, we show that fluid shear stress increases the expression of Runx1 in CD41+c-Kit+ haematopoietic progenitor cells7, concomitantly augmenting their haematopoietic colony-forming potential. Moreover, we find that shear stress increases haematopoietic colony-forming potential and expression of haematopoietic markers in the para-aortic splanchnopleura/aorta–gonads–mesonephros of mouse embryos and that abrogation of nitric oxide, a mediator of shear-stress-induced signalling8, compromises haematopoietic potential in vitro and in vivo. Collectively, these data reveal a critical role for biomechanical forces in haematopoietic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hove, J. R. et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172–177 (2003)
Lucitti, J. L. et al. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134, 3317–3326 (2007)
Garcia-Porrero, J. A., Godin, I. E. & Dieterlen-Lievre, F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat. Embryol. 192, 425–435 (1995)
North, T. E. et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16, 661–672 (2002)
Rhodes, K. E. et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2, 252–263 (2008)
Lensch, M. W. & Daley, G. Q. Origins of mammalian hematopoiesis: in vivo paradigms and in vitro models. Curr. Top. Dev. Biol. 60, 127–196 (2004)
Mikkola, H. K., Fujiwara, Y., Schlaeger, T. M., Traver, D. & Orkin, S. H. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 101, 508–516 (2003)
Garcia-Cardena, G. et al. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392, 821–824 (1998)
Haar, J. L. & Ackerman, G. A. A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat. Rec. 170, 199–223 (1971)
Ji, R. P. et al. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ. Res. 92, 133–135 (2003)
Tavian, M. et al. The vascular wall as a source of stem cells. Ann. NY Acad. Sci. 1044, 41–50 (2005)
Garin, G. & Berk, B. C. Flow-mediated signaling modulates endothelial cell phenotype. Endothelium 13, 375–384 (2006)
Kabrun, N. et al. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development 124, 2039–2048 (1997)
Lengerke, C. et al. BMP and WNT specify hematopoietic fate by activation of the CDX-Hox pathway. Cell Stem Cell 2, 72–82 (2008)
Medvinsky, A. & Dzierzak, E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86, 897–906 (1996)
Cumano, A., Dieterlen-Lievre, F. & Godin, I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86, 907–916 (1996)
Phoon, C. K., Aristizabal, O. & Turnbull, D. H. Spatial velocity profile in mouse embryonic aorta and Doppler-derived volumetric flow: a preliminary model. Am. J. Physiol. Heart Circ. Physiol. 283, H908–H916 (2002)
Ku, D. Blood flow in arteries. Annu. Rev. Fluid Mech. 29, 399–434 (1997)
Yamamoto, K. et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro . Am. J. Physiol. Heart Circ. Physiol. 288, H1915–H1924 (2005)
Basu, P. et al. KLF2 is essential for primitive erythropoiesis and regulates the human and murine embryonic beta-like globin genes in vivo . Blood 106, 2566–2571 (2005)
Parmar, K. M. et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116, 49–58 (2006)
Wang, Y., Yates, F., Naveiras, O., Ernst, P. & Daley, G. Q. Embryonic stem cell-derived hematopoietic stem cells. Proc. Natl Acad. Sci. USA 102, 19081–19086 (2005)
Aicher, A. et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nature Med. 9, 1370–1376 (2003)
Rees, D. D., Palmer, R. M. & Moncada, S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl Acad. Sci. USA 86, 3375–3378 (1989)
Ferkowicz, M. J. et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development 130, 4393–4403 (2003)
Koushik, S. V. et al. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J. 15, 1209–1211 (2001)
Lux, C. T. et al. All primitive and definitive hematopoietic progenitor cells emerging prior to E10 in the mouse embryo are products of the yolk sac. Blood 111, 3435–3438 (2007)
Simeone, A., Daga, A. & Calabi, F. Expression of runt in the mouse embryo. Dev. Dyn. 203, 61–70 (1995)
North, T. et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126, 2563–2575 (1999)
Jones, E. A., Baron, M. H., Fraser, S. E. & Dickinson, M. E. Measuring hemodynamic changes during mammalian development. Am. J. Physiol. Heart Circ. Physiol. 287, H1561–H1569 (2004)
Ji, R. P. et al. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ. Res. 92, 133–135 (2003)
Phoon, C. K., Aristizabal, O. & Turnbull, D. H. Spatial velocity profile in mouse embryonic aorta and Doppler-derived volumetric flow: a preliminary model. Am. J. Physiol. Heart Circ. Physiol. 283, H908–H916 (2002)
Jones, E. A., Baron, M. H., Fraser, S. E. & Dickinson, M. E. Measuring hemodynamic changes during mammalian development. Am. J. Physiol. Heart Circ. Physiol. 287, H1561–H1569 (2004)
Nosek, T. M. Essentials of Human physiology — Cardiac and Circulatory Physiology (Gold Standard Multimedia, 2000)
Blackman, B. R., Garcia-Cardena, G. & Gimbrone, M. A. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J. Biomech. Eng. 124, 397–407 (2002)
Wang, Y., Yates, F., Naveiras, O., Ernst, P. & Daley, G. Q. Embryonic stem cell-derived hematopoietic stem cells. Proc. Natl Acad. Sci. USA 102, 19081–19086 (2005)
Tiboni, G. M., Marotta, F. & Barbacane, L. Production of axial skeletal malformations with the nitric oxide synthesis inhibitor NG-nitro-l-arginine methyl ester (L-NAME) in the mouse. Birth Defects Res. B Dev. Reprod. Toxicol. 80, 28–33 (2007)
Acknowledgements
We thank G. Losyev for assistance with flow cytometry, S. Schmitt for critical help in optimizing AGM culture conditions, C. Lengerke and Y. Mukouyama for critical discussions. L.A. was partially funded by the Giovanni Armenise-Harvard Foundation. O.N. was partially funded by the Barrie de la Maza Foundation. G.G.-C. was supported by grants from the National Institutes of Health and G.Q.D was supported by grants from the National Institutes of Health (NIH), and the NIH Director’s Pioneer Award of the NIH Roadmap for Medical Research. G.Q.D. is a recipient of the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research and is an Investigator of the Howard Hughes Medical Institute.
Author Contributions L.A., O.N, G.G.-C. and G.Q.D. conceived ideas, designed experiments, analysed results and wrote the manuscript. P.J.M. performed the haemodynamic shear stress estimation and programmed the biomechanical stimuli. L.A., O.N., P.L.W., J.G.-S., S.M.-F. and A.S.-D. performed experiments. M.W.L. conceived ideas and contributed to experimental design. M.Y. and M.C.Y. set up timed pregnancies and isolated Ncx1 null and wild-type mouse embryos. All authors edited and reviewed the final manuscript. G.Q.D. and G.G.-C. co-directed the project.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Figures
This file contains Supplementary Figures S1-S3 with Legends. (PDF 699 kb)
Rights and permissions
About this article
Cite this article
Adamo, L., Naveiras, O., Wenzel, P. et al. Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131–1135 (2009). https://doi.org/10.1038/nature08073
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08073
This article is cited by
-
The spatiotemporal heterogeneity of the biophysical microenvironment during hematopoietic stem cell development: from embryo to adult
Stem Cell Research & Therapy (2023)
-
Generating hematopoietic cells from human pluripotent stem cells: approaches, progress and challenges
Cell Regeneration (2023)
-
Spatio-temporal dynamics enhance cellular diversity, neuronal function and further maturation of human cerebral organoids
Communications Biology (2023)
-
Hydrogel-based microenvironment engineering of haematopoietic stem cells
Cellular and Molecular Life Sciences (2023)
-
Interplay between mechanics and signalling in regulating cell fate
Nature Reviews Molecular Cell Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.