Abstract
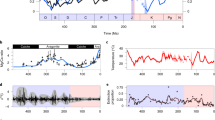
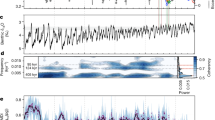
Diatoms are the dominant group of phytoplankton in the modern ocean. They account for approximately 40% of oceanic primary productivity and over 50% of organic carbon burial in marine sediments1. Owing to their role as a biological carbon pump2 and effects on atmospheric CO2 levels3,4,5, there is great interest in elucidating factors that influenced the rapid rise in diatom diversity during the past 40 million years6,7. Two biotic controls on diversification have been proposed to explain this diversity increase: (1) geochemical coupling between terrestrial grasslands and marine ecosystems through the global silicon cycle; and (2) competitive displacement of other phytoplankton lineages. However, these hypotheses have not been tested using sampling-standardized fossil data. Here we show that reconstructions of species diversity in marine phytoplankton reject these proposed controls and suggest a new pattern for oceanic diatom diversity across the Cenozoic. Peak species diversity in marine planktonic diatoms occurred at the Eocene–Oligocene boundary and was followed by a pronounced decline, from which diversity has not recovered. Although the roles of abiotic and biotic drivers of diversification remain unclear, major features of oceanic diatom evolution are decoupled from both grassland expansion and competition among phytoplankton groups.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Falkowski, P. G. et al. The evolution of modern eukaryotic phytoplankton. Science 305, 354–360 (2004)
Treguer, P. & Pondaven, P. Global change – silica control of carbon dioxide. Nature 406, 358–359 (2000)
Falkowski, P. G., Barber, R. T. & Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 281, 200–206 (1998)
Milligan, A. J. & Morel, F. M. M. A proton buffering role for silica in diatoms. Science 297, 1848–1850 (2002)
Katz, M. E. et al. Biological overprint of the geological carbon cycle. Mar. Geol. 217, 323–338 (2005)
Falkowski, P. G., Schofield, O., Katz, M. E., Van De Schootbrugge, B. & Knoll, A. H. in Coccolithophores: From Molecular Processes to Global Impact (eds Therstein, H. R. & Young, J. R.) 429–454 (Springer, 2004)
Finkel, Z. V., Katz, M. E., Wright, J. D., Schofield, O. M. E. & Falkowski, P. G. Climatically driven macroevolutionary patterns in the size of marine diatoms over the Cenozoic. Proc. Natl Acad. Sci. USA 201, 8927–8932 (2005)
Kidder, D. L. & Gierlowski-Kordesch, E. H. Impact of grassland radiation on the nonmarine silica cycle and Miocene diatomite. Palaios 20, 198–206 (2005)
Kidder, D. L. & Erwin, D. H. Secular distribution of biogenic silica through the phanerozoic: comparison of silica-replaced fossils and bedded cherts at the series level. J. Geol. 109, 509–522 (2001)
Blecker, S. W., McCulley, R. L., Chadwick, O. A. & Kelly, E. F. Biologic cycling of silica across a grassland bioclimosequence. Glob. Biogeochem. Cycles 20, GB3023 (2006)
Spencer-Cervato, C. The Cenozoic deep-sea microfossil record: explorations of the DSDP/ODP sample set using the Neptune database. Palaeontol. Electron. 2, 1–268 (1999)
Alroy, J. et al. Effects of sampling standardization on estimates of Phanerozoic marine diversification. Proc. Natl Acad. Sci. USA 98, 6261–6266 (2001)
Raup, D. M. Taxonomic diversity during the Phanerozoic. Science 177, 1065–1071 (1972)
Alroy, J. The dynamics of origination and extinction in the marine fossil record. Proc. Natl Acad. Sci. USA 105, 11536–11542 (2008)
Miller, A. I. & Foote, M. Calibrating the Ordovician radiation of marine life: implications for Phanerozoic diversity trends. Paleobiology 22, 304–309 (1996)
Smith, E. P., Stewart, P. M. & Cairns, J. Similarities between rarefaction methods. Hydrobiologia 120, 167–170 (1985)
Alroy, J. New methods for quantifying macroevolutionary patterns and processes. Paleobiology 26, 707–733 (2000)
Stromberg, C. A. E. Decoupled taxonomic radiation and ecological expansion of open-habitat grasses in the Cenozoic of North America. Proc. Natl Acad. Sci. USA 102, 11980–11984 (2005)
Retallack, G. J. Cenozoic expansion of grasslands and climatic cooling. J. Geol. 109, 407–426 (2001)
MacFadden, B. J. Fossil Horses (Cambridge Univ. Press, 1992)
Margalef, R. Our Biosphere (Ecology Institute, 1997)
Sellner, K. G., Sellner, S. G., Lacouture, R. V. & Magnien, R. E. Excessive nutrients select for dinoflagellates in the stratified Patapsco River estuary: Margalef reigns. Mar. Ecol. Prog. Ser. 220, 93–102 (2001)
Tozzi, S., Schofield, O. & Falkowski, P. Historical climate change and ocean turbulence as selective agents for two key phytoplankton functional groups. Mar. Ecol. Prog. Ser. 274, 123–132 (2004)
Iglesias-Rodriguez, M. D. et al. Representing key phytoplankton functional groups in ocean carbon cycle models: coccolithophorids. Glob. Biogeochem. Cycles 16, 1100 (2002)
Bown, P. R. Calcareous nannoplankton evolution: a tale of two oceans. Micropaleontology 51, 299–308 (2005)
Katz, M. E., Finkel, Z. V., Grzebyk, D., Knoll, A. H. & Falkowski, P. G. Evolutionary trajectories and biogeochemical impacts of marine eukaryotic phytoplankton. Annu. Rev. Ecol. Evol. Syst. 35, 523–556 (2004)
Prothero, D. R., Ivany, L. C. & Nesbitt, E. A. (eds) From Greenhouse to Icehouse: The Marine Eocene–Oligocene Transition (Columbia Univ. Press, 2003)
Zanazzi, A., Kohn, M. J., MacFadden, B. J. & Terry, D. O. Large temperature drop across the Eocene–Oligocene transition in central North. Am. Nat. 445, 639–642 (2007)
Pagani, M., Zachos, J. C., Freeman, K. H., Tipple, B. & Bohaty, S. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science 309, 600–603 (2005)
Sepkoski, J. J. A factor analytic description of the Phanerozoic marine fossil record. Paleobiology 7, 36–53 (1981)
Bush, A. M., Markey, M. J. & Marshall, C. R. Removing bias from diversity curves: the effects of spatially organized biodiversity on sampling-standardization. Paleobiology 30, 666–686 (2004)
Alroy, J. et al. Phanerozoic trends in the diversity of marine invertebrates. Science 321, 97–100 (2008)
Paul, C. R. C. in The Adequacy of the Fossil Record (eds Donovan, S. K. & Paul, C. R. C.) 1–22 (Wiley, 1982)
Foote, M. Morphological disparity in Ordovician–Devonian crinoids and the early saturation of morphological space. Paleobiology 20, 320–344 (1994)
Acknowledgements
We thank J. Alroy for comments on our statistical analyses and for suggesting the binomial approximation for confidence limits on diversity; and M. Foote, I. Lovette, A. McCune, A. Agrawal, N. Hairston and the BK Discussion Group for comments on the manuscript. This research was supported in part by NSF-OSIEE-0612855. Part of this work used the resources of the Computational Biology Service Unit from Cornell University, which is partly funded by the Microsoft Corporation.
Author Contributions Both authors contributed to study design and the interpretation of results. D.L.R. conducted the analyses and drafted the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures S1-S1- with Legends (PDF 1683 kb)
Rights and permissions
About this article
Cite this article
Rabosky, D., Sorhannus, U. Diversity dynamics of marine planktonic diatoms across the Cenozoic. Nature 457, 183–186 (2009). https://doi.org/10.1038/nature07435
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07435
This article is cited by
-
Degradation of biomass tar catalyzed by Fe/Ce supported on diatomite-based foam ceramics
Biomass Conversion and Biorefinery (2023)
-
A New Machine-Learning Extracting Approach to Construct a Knowledge Base: A Case Study on Global Stromatolites over Geological Time
Journal of Earth Science (2023)
-
Combining palaeontological and neontological data shows a delayed diversification burst of carcharhiniform sharks likely mediated by environmental change
Scientific Reports (2022)
-
The exceptional preservation of Aix-en-Provence spider fossils could have been facilitated by diatoms
Communications Earth & Environment (2022)
-
Incorporating a molecular antenna in diatom microalgae cells enhances photosynthesis
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.