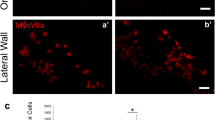
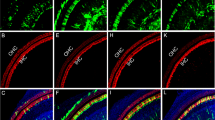
Abstract
Sensory hair cells in the mammalian cochlea convert mechanical stimuli into electrical impulses that subserve audition1,2. Loss of hair cells and their innervating neurons is the most frequent cause of hearing impairment3. Atonal homologue 1 (encoded by Atoh1, also known as Math1) is a basic helix–loop–helix transcription factor required for hair-cell development4,5,6, and its misexpression in vitro7,8 and in vivo9,10 generates hair-cell-like cells. Atoh1-based gene therapy to ameliorate auditory10 and vestibular11 dysfunction has been proposed. However, the biophysical properties of putative hair cells induced by Atoh1 misexpression have not been characterized. Here we show that in utero gene transfer of Atoh1 produces functional supernumerary hair cells in the mouse cochlea. The induced hair cells display stereociliary bundles, attract neuronal processes and express the ribbon synapse marker carboxy-terminal binding protein 2 (refs 12,13). Moreover, the hair cells are capable of mechanoelectrical transduction1,2 and show basolateral conductances with age-appropriate specializations. Our results demonstrate that manipulation of cell fate by transcription factor misexpression produces functional sensory cells in the postnatal mammalian cochlea. We expect that our in utero gene transfer paradigm will enable the design and validation of gene therapies to ameliorate hearing loss in mouse models of human deafness14,15.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vollrath, M., Kwan, K. & Corey, D. The micromachinery of mechanotransduction in hair cells. Annu. Rev. Neurosci. 30, 339–365 (2007)
Grant, L. & Fuchs, P. A. Auditory transduction in the mouse. Pflugers Arch. 454, 793–804 (2007)
Davis, A. in Hearing Science and Hearing Disorders (eds Lutman, M. & Haggard, M.) (Academic, 1993)
Bermingham, N. A. et al. Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841 (1999)
Chen, P., Johnson, J. E., Zoghbi, H. Y. & Segil, N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129, 2495–2505 (2002)
Jones, J. M. et al. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J. Neurosci. 26, 550–558 (2006)
Zheng, J. L. & Gao, W. Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nature Neurosci. 3, 580–586 (2000)
Woods, C., Montcouquiol, M. & Kelley, M. W. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nature Neurosci. 7, 1310–1318 (2004)
Kawamoto, K. et al. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo . J. Neurosci. 23, 4395–4400 (2003)
Izumikawa, M. et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature Med. 11, 271–276 (2005)
Staecker, H., Praetorius, M., Baker, K. & Brough, D. E. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol. Neurotol. 28, 223–231 (2007)
Knirsch, M. et al. Persistence of Cav1.3 Ca2+ channels in mature outer hair cells supports outer hair cell afferent signaling. J. Neurosci. 27, 6442–6451 (2007)
Wan, L., Almers, W. & Chen, W. Two ribeye genes in teleosts: the role of Ribeye in ribbon formation and bipolar cell development. J. Neurosci. 25, 941–949 (2005)
Brown, S. D., Hardisty-Hughes, R. E. & Mburu, P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nature Rev. Genet. 9, 277–290 (2008)
Friedman, L. M., Dror, A. A. & Avraham, K. B. Mouse models to study inner ear development and hereditary hearing loss. Int. J. Dev. Biol. 51, 609–631 (2007)
Gubbels, S., Woessner, D., Mitchell, J. & Brigande, J. in Thirtieth Annual MidWinter Research Meeting of the Association for Research in Otolaryngology, 10–15 February 2007 (ed. Santi, P. A.) vol. 30 330 (Association for Research in Otolaryngology, 2007)
Kim, D. W. et al. Use of the human elongation factor 1α promoter as a versatile and efficient expression system. Gene 91, 217–223 (1990)
Li, X. et al. Generation of destabilized green fluorescent protein as a transcription reporter. J. Biol. Chem. 273, 34970–34975 (1998)
Rechsteiner, M. PEST sequences are signals for rapid intracellular proteolysis. Semin. Cell Biol. 1, 433–440 (1990)
Sher, A. E. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol. Suppl. 285, 1–77 (1971)
Hasson, T. et al. Unconventional myosins in inner ear sensory epithelia. J. Cell Biol. 137, 1287–1307 (1997)
Hasson, T. et al. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc. Natl Acad. Sci. USA 92, 9815–9819 (1995)
Chen, P. & Segil, N. p27Kip1 links cell proliferation to morphogenesis in the developing organ of Corti. Development 126, 1581–1590 (1999)
Maklad, A. & Fritzsch, B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Brain Res. Dev. Brain Res. 140, 223–236 (2003)
Fritzsch, B. et al. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue Maroon, NeuroVue Red and NeuroVue Green and their use for double and triple labeling of neuronal profile. Brain Res. Bull. 66, 249–258 (2005)
Waguespack, J., Salles, F. T., Kachar, B. & Ricci, A. J. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J. Neurosci. 27, 13890–13902 (2007)
Wu, Y. C., Ricci, A. J. & Fettiplace, R. Two components of transducer adaptation in auditory hair cells. J. Neurophysiol. 82, 2171–2181 (1999)
Li, H., Corrales, C. E., Edge, A. & Heller, S. Stem cells as therapy for hearing loss. Trends Mol. Med. 10, 309–315 (2004)
Hu, Z. & Ulfendahl, M. Cell replacement therapy in the inner ear. Stem Cells Dev. 15, 449–459 (2006)
Self, T. et al. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development 125, 557–566 (1998)
Acknowledgements
We thank C. Bresee and J. Jungwirth for expert technical support; C. Cepko and G. Nolan for plasmids EF1α–GFP and BMN–IRES–GFP, respectively; A. Kiernan, D. Fekete, S. Heller, A. Nguyen-Huynh, C. Bresee and J. Jungwirth for critical comments that improved the manuscript; D. Trune, B. Fritzsch and N. Segil for helpful discussions; S. Griest for statistical analyses; and M. Campbell and S. Nigra for exceptional animal care. This study was supported by grants from the National Institute on Deafness and Other Communication Disorders (J.V.B. and A.J.R.), the McKnight Endowment Fund for Neuroscience (J.J.B.) and the American Otological Society (Research Training Fellowship to S.P.G.).
Author Contributions The project was conceived by J.V.B. Experiments were planned and performed by S.P.G. and D.W.W. with advice from J.V.B., and were analysed by S.P.G., D.W.W. and J.V.B. J.V.B. performed the experimental embryology. A.J.R. conducted the electrophysiology experiments and interpreted the results. J.C.M. acquired the scanning electron micrographs. J.V.B. and A.J.R. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-4; Supplementary Tables 1 and 2; Supplementary Video Legends 1-4; and a discussion of the efficacy of transuterine microinjection and in vivo electroporation. (PDF 540 kb)
Supplementary Video 1
This file contains Supplementary Video 1 (MOV 2491 kb)
Supplementary Video 2
This file contains Supplementary Video 2 (MOV 10550 kb)
Supplementary Video 3
This file contains Supplementary Video 3 (MOV 6183 kb)
Supplementary Video 4
This file contains Supplementary Video 4 (MOV 2441 kb)
Rights and permissions
About this article
Cite this article
Gubbels, S., Woessner, D., Mitchell, J. et al. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 455, 537–541 (2008). https://doi.org/10.1038/nature07265
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07265
This article is cited by
-
Stem Cell-Based Hair Cell Regeneration and Therapy in the Inner Ear
Neuroscience Bulletin (2024)
-
Differential regulation of mammalian and avian ATOH1 by E2F1 and its implication for hair cell regeneration in the inner ear
Scientific Reports (2021)
-
Combinatorial Atoh1 and Gfi1 induction enhances hair cell regeneration in the adult cochlea
Scientific Reports (2020)
-
Prenatal electroporation-mediated gene transfer restores Slc26a4 knock-out mouse hearing and vestibular function
Scientific Reports (2019)
-
Strategien für eine regenerative Therapie der Schwerhörigkeit
HNO (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.