Abstract
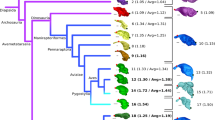
All adult flatfishes (Pleuronectiformes), including the gastronomically familiar plaice, sole, turbot and halibut, have highly asymmetrical skulls, with both eyes placed on one side of the head. This arrangement, one of the most extraordinary anatomical specializations among vertebrates, arises through migration of one eye during late larval development. Although the transformation of symmetrical larvae into asymmetrical juveniles is well documented1,2,3,4,5,6,7, the evolutionary origins of flatfish asymmetry are uncertain1,2 because there are no transitional forms linking flatfishes with their symmetrical relatives8,9. The supposed inviability of such intermediates gave pleuronectiforms a prominent role in evolutionary debates10,11,12,13,14,15,16, leading to attacks on natural selection11 and arguments for saltatory change14,15. Here I show that Amphistium and the new genus Heteronectes, both extinct spiny-finned fishes from the Eocene epoch of Europe, are the most primitive pleuronectiforms known. The orbital region of the skull in both taxa is strongly asymmetrical, as in living flatfishes, but these genera retain many primitive characters unknown in extant forms. Most remarkably, orbital migration was incomplete in Amphistium and Heteronectes, with eyes remaining on opposite sides of the head in post-metamorphic individuals. This condition is intermediate between that in living pleuronectiforms and the arrangement found in other fishes. Amphistium and Heteronectes indicate that the evolution of the profound cranial asymmetry of extant flatfishes was gradual in nature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kyle, H. M. The asymmetry, metamorphosis, and origin of flat-fishes. Phil. Trans. R. Soc. Lond. B 211, 75–129 (1923)
Norman, J. R. A Systematic Monograph of the Flatfishes (Heterosomata). Vol. 1. Psettotidae, Bothidae, Pleuronectidae (British Museum (Natural History), London, 1934)
Ahlstrom, E. H., Amaoka, K., Hensley, D. A., Moser, H. G. & Sumida, B. Y. in Ontogeny and Systematics of Fishes (eds Moser, H. G. et al.) 640–670 (Allen, Lawrence, Kansas, 1984)
Okada, N., Takagi, Y., Seikai, T., Tanaka, M. & Tagawa, M. Asymmetrical development of bones and soft tissues during eye migration of metamorphosing Japanese flounder, Paralichthys olivaceus. Cell Tissue Res. 304, 59–66 (2001)
Wagemans, F. & Vandewalle, P. Development of the bony skull in common sole: brief survey of morpho-functional aspects of ossification sequence. J. Fish Biol. 59, 1350–1369 (2001)
Hoshino, K. Fixing the confused term “pseudomesial bar” and homologies of pleuronectiform cranial elements, with proposals of new terms. Ichthyol. Res. 53, 435–440 (2006)
Schreiber, A. M. Asymmetric craniofacial remodeling and lateralized behavior in larval flatfish. J. Exp. Biol. 209, 610–621 (2006)
Chanet, B. A cladistic reappraisal of the fossil flatfishes record consequences on the phylogeny of the Pleuronectiformes (Osteichthyes: Teleostei). Ann. Sci. Nat. Zool. 18, 105–117 (1997)
Chanet, B. Supposed and true flatfishes [Teleostei: Pleuronectiformes] from the Eocene of Monte Bolca, Italy. Stud. Ric. Giacim. Terz. Bolca 8, 220–243 (1999)
Lamarck, J. B. Philosophie Zoologique (Dentu, Paris, 1809)
Mivart, G. J. On the Genesis of Species (MacMillan, London, 1871)
Darwin, C. On the Origin of Species by Means of Natural Selection 6th edn (Murray, London, 1872)
Wallace, A. R. Darwinism (MacMillan and Co., London, 1889)
Goldschmidt, R. Some aspects of evolution. Science 78, 539–547 (1933)
Goldschmidt, R. The Material Basis of Evolution (Yale Univ. Press, New Haven, 1940)
von Wahlert, G. The role of ecological factors in the origin of higher levels of organization. Syst. Zool. 14, 288–300 (1965)
Chanet, B. Eubuglossus eocenicus (Woodward 1910) from the Upper Lutetian of Egypt, one of the oldest soleids (Teleostei, Pleuronectiformes). Neues Jahrb. Geol. Paläntol. Monatsh. 1994, 391–398 (1994)
Trevisani, E., Papazzoni, C. A., Ragazzi, E. & Roghi, G. Early Eocene amber from the “Pesciara di Bolca” (Lessini Mountains, Northern Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 223, 260–274 (2005)
Chapleau, F. Pleuronectiform relationships: a cladistic reassessment. Bull. Mar. Sci. 52, 516–540 (1993)
Blot, J. Les poissons fossiles du Monte Bolca. Classés jusqu’ici dans les familles des Carangidae, Menidae, Ephippidae, Scatophagidae. Mem. Mus. Civ. Stor. Nat. Verona 1, 1–525 (1969)
Gaudant, J. Sur un Amphistium (poisson téléostéen, Percoidei) de l’Éocène Parisien. Bull. Inf. Géol. Bass. Paris 16, 11–14 (1979)
Liew, H.-C., Milward, N. E. & Hartwick, R. F. Descriptions of larval fishes of the genera Psettodes (Psettotidae) and Pseuorhombus (Paralichthyidae) from the Great Barrier Reef, Australia. Aust. J. Mar. Freshwat. Res. 39, 51–70 (1988)
Gosline, W. A. The perciform caudal skeleton. Copeia 1961, 265–270 (1961)
Patterson, C. An overview of the early fossil record of acanthomorphs. Bull. Mar. Sci. 52, 29–59 (1993)
Johnson, G. D. & Patterson, C. Percomorph phylogeny: a survey of acanthomorphs and a new proposal. Bull. Mar. Sci. 52, 554–626 (1993)
Hubbs, C. L. & Hubbs, L. C. Bilateral asymmetry and bilateral variation in fishes. Pap. Mich. Acad. Sci. Arts Lett. 30, 229–310 (1944)
Palmer, A. R. From symmetry to asymmetry: phylogenetic patterns of asymmetry variation in animals and their evolutionary significance. Proc. Natl Acad. Sci. USA 93, 14279–14286 (1996)
Stickney, R. R., White, D. B. & Miller, D. Observations of fin use in relation to feeding and resting behavior in flatfishes (Pleuronectiformes). Copeia 1973, 154–156 (1973)
Patterson, C. in The Fossil Record 2 (ed. Benton, M. J.) 621–656 (Chapman & Hall, London, 1993)
Stiassny, M. L. J., Wiley, E. O., Johnson, G. D. & de Carvalho, M. R. in Assembling the Tree of Life (eds Cracraft, J. & Donoghue, M. J.) 410–429 (Oxford Univ. Press, New York, 2004)
Acknowledgements
I thank G. Clément, P. Forey, D. Goujet, M. Richter, O. Schultz, A. Vaccari and M. Véran for loaning or providing access to fossil specimens; R. Arrindell, B. Brown, D. Johnson, S. Raredon, M. Rogers and M. Westneat for arranging the loan or study of recent material; E. Hilton for checking gill-arch characters in carangids; M. Colbert and A. Gosselin-Ildari for compiling the computed tomography renderings, K. Claeson for providing specimen transport; L. Herzog, A. Shinya, D. Wagner and J. Holstein for helping in fossil preparation; and A. Bannikov, M. Coates, M. LaBarbera and N. Smith for discussion. This work was supported by a grant from the Lerner-Grey Fund for Marine Research, a Hinds Fund Grant, an Evolving Earth Grant, a National Science Foundation Graduate Research Fellowship (award number DGE-0228235), and an Environmental Protection Agency STAR Fellowship (award number FP916730).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information
The file contains Supplementary Information which includes the following sections: I. Institutional abbreviations; II. Supplementary morphological data: †Heteronectes chaneti; III. Details of computed tomography (CT); IV. Supplementary morphological data: †Amphistium; V. Legends for CT rendering movies; VI. Characters used in cladistic analysis; VII. Comparative materials used to code characters: fossil taxa; VIII. Comparative materials used to code characters: Recent taxa; IX. Taxon-by-character matrix; X. Systematic methodology; XI. Character optimisations; XII. additional references.. The file also includes Supplementary Figures 1-20 with Legends. (PDF 38916 kb)
Supplementary information
The file contains Supplementary Movie 1 showing CT rendering of neurocranium of †Amphistium paradoxum (BMNH P.16138; dextral morph). Animation begins with neurocranium in right-lateral view (damaged side with unmigrated orbit; the skull was fractured when the slab containing the fish was split), then rotates the skull 360 degrees about a vertical axis. Upon returning to start position, the skull rotates the skull 360 degrees about its long axis, showing ventral, left-lateral (side with migrated orbit), and dorsal views. Specimen missing ethmoid region and parasphenoid (broken off block). A still image of the CT rendering and interpretive line drawing are given in Supplementary figure 10. A photograph of the complete specimen is shown in Supplementary figure 8, and a close-up of the skull in Supplementary figure 9. (MOV 1619 kb)
Supplementary information
The file contains Supplementary Movie 2 showing CT rendering of neurocranium of †Amphistium paradoxum (BMNH P.1982; dextral morph). Animation begins with neurocranium in right-lateral view (damaged side with unmigrated orbit; the skull was fractured when the slab containing the fish was split), then rotates the skull 360 degrees about a vertical axis. Upon returning to start position, the skull rotates the skull 360 degrees about its long axis showing ventral, left-lateral (side with migrated orbit), and dorsal views. The latter clearly shows medial extent of migrated orbit. A still image of the CT rendering and interpretive line drawing are given in Supplementary figure (MOV 1359 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Friedman, M. The evolutionary origin of flatfish asymmetry. Nature 454, 209–212 (2008). https://doi.org/10.1038/nature07108
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07108
This article is cited by
-
Unraveling the transcriptomic landscape of eye migration and visual adaptations during flatfish metamorphosis
Communications Biology (2024)
-
Evolutionary ecology of the visual opsin gene sequence and its expression in turbot (Scophthalmus maximus)
BMC Ecology and Evolution (2021)
-
Righting behaviour in the European pond turtle (Emys orbicularis): relations between behavioural and morphological lateralization
Animal Cognition (2020)
-
Internal embryonic development in a non-copulatory, egg-laying teleost, the three-spined stickleback, Gasterosteus aculeatus
Scientific Reports (2019)
-
A bizarre Eocene dasyatoid batomorph (Elasmobranchii, Myliobatiformes) from the Bolca Lagerstätte (Italy) reveals a new, extinct body plan for stingrays
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.