Abstract
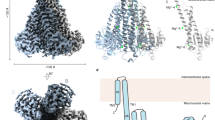
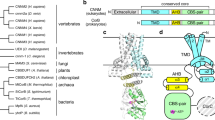
The magnesium ion Mg2+ is a vital element involved in numerous physiological processes. Mg2+ has the largest hydrated radius among all cations, whereas its ionic radius is the smallest. It remains obscure how Mg2+ transporters selectively recognize and dehydrate the large, fully hydrated Mg2+ cation for transport1. Recently the crystal structures of the CorA Mg2+ transporter2,3,4,5 were reported6,7,8. The MgtE family of Mg2+ transporters is ubiquitously distributed in all phylogenetic domains9,10,11, and human homologues have been functionally characterized and suggested to be involved in magnesium homeostasis12,13,14. However, the MgtE transporters have not been thoroughly characterized. Here we determine the crystal structures of the full-length Thermus thermophilus MgtE at 3.5 Å resolution, and of the cytosolic domain in the presence and absence of Mg2+ at 2.3 Å and 3.9 Å resolutions, respectively. The transporter adopts a homodimeric architecture, consisting of the carboxy-terminal five transmembrane domains and the amino-terminal cytosolic domains, which are composed of the superhelical N domain and tandemly repeated cystathionine-β-synthase domains. A solvent-accessible pore nearly traverses the transmembrane domains, with one potential Mg2+ bound to the conserved Asp 432 within the pore. The transmembrane (TM)5 helices from both subunits close the pore through interactions with the ‘connecting helices’, which connect the cystathionine-β-synthase and transmembrane domains. Four putative Mg2+ ions are bound at the interface between the connecting helices and the other domains, and this may lock the closed conformation of the pore. A structural comparison of the two states of the cytosolic domains showed the Mg2+-dependent movement of the connecting helices, which might reorganize the transmembrane helices to open the pore. These findings suggest a homeostasis mechanism, in which Mg2+ bound between cytosolic domains regulates Mg2+ flux by sensing the intracellular Mg2+ concentration. Whether this presumed regulation controls gating of an ion channel or opening of a secondary active transporter remains to be determined.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Maguire, M. E. Magnesium transporters: properties, regulation and structure. Front. Biosci. 11, 3149–3163 (2006)
Nelson, D. L. & Kennedy, E. P. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J. Biol. Chem. 246, 3042–3049 (1971)
Hmiel, S. P., Snavely, M. D., Miller, C. G. & Maguire, M. E. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 168, 1444–1450 (1986)
Bui, D. M., Gregan, J., Jarosch, E., Ragnini, A. & Schweyen, R. J. The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondrial membrane. J. Biol. Chem. 274, 20438–20443 (1999)
Kehres, D. G. & Maguire, M. E. Structure, properties and regulation of magnesium transport proteins. Biometals 15, 261–270 (2002)
Lunin, V. V. et al. Crystal structure of the CorA Mg2+ transporter. Nature 440, 833–837 (2006)
Eshaghi, S. et al. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science 313, 354–357 (2006)
Payandeh, J. & Pai, E. F. A structural basis for Mg2+ homeostasis and the CorA translocation cycle. EMBO J. 25, 3762–3773 (2006)
Townsend, D. E. et al. Cloning of the mgtE Mg2+ transporter from Providencia stuartii and the distribution of mgtE in Gram-negative and Gram-positive bacteria. J. Bacteriol. 177, 5350–5354 (1995)
Smith, R. L., Thompson, L. J. & Maguire, M. E. Cloning and characterization of MgtE, a putative new class of Mg2+ transporter from Bacillus firmus OF4. J. Bacteriol. 177, 1233–1238 (1995)
Wabakken, T., Rian, E., Kveine, M. & Aasheim, H. C. The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochem. Biophys. Res. Commun. 306, 718–724 (2003)
Goytain, A. & Quamme, G. A. Functional characterization of human SLC41A1, a Mg2+ transporter with similarity to prokaryotic MgtE Mg2+ transporters. Physiol. Genomics 21, 337–342 (2005)
Goytain, A. & Quamme, G. A. Functional characterization of the human solute carrier, SLC41A2. Biochem. Biophys. Res. Commun. 330, 701–705 (2005)
Sahni, J., Nelson, B. & Scharenberg, A. M. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem. J. 401, 505–513 (2007)
Kowal, P., Gurtan, A. M., Stuckert, P., D'Andrea, A. D. & Ellenberger, T. Structural determinants of human FANCF protein that function in the assembly of a DNA damage signaling complex. J. Biol. Chem. 282, 2047–2055 (2007)
Ignoul, S. & Eggermont, J. CBS domains: structure, function, and pathology in human proteins. Am. J. Physiol. Cell Physiol. 289, C1369–C1378 (2005)
Bennetts, B. et al. Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J. Biol. Chem. 280, 32452–32458 (2005)
Biemans-Oldehinkel, E., Mahmood, N. A. & Poolman, B. A sensor for intracellular ionic strength. Proc. Natl Acad. Sci. USA 103, 10624–10629 (2006)
Tieleman, D. P., Shrivastava, I. H., Ulmschneider, M. R. & Sansom, M. S. Proline-induced hinges in transmembrane helices: possible roles in ion channel gating. Proteins 44, 63–72 (2001)
Roux, B. & MacKinnon, R. The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science 285, 100–102 (1999)
Kuo, A. et al. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300, 1922–1926 (2003)
Hattori, M., Tanaka, Y., Fukai, S., Ishitani, R. & Nureki, O. Crystallization and preliminary X-ray diffraction analysis of the full-length Mg2+ transporter MgtE. Acta Crystallogr. F 63, 682–684 (2007)
Tanaka, Y., Hattori, M., Fukai, S., Ishitani, R. & Nureki, O. Crystallization and preliminary X-ray diffraction analysis of the cytosolic domain of the Mg2+ transporter MgtE. Acta Crystallogr. F 63, 678–681 (2007)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Collaborative Computational Project 4 The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Weeks, C. M. & Miller, R. The design and implementation of SnB v2.0. J. Appl. Cryst. 32, 120–124 (1999)
de La Fortelle, E., Irwin, J. & Bricogne, G. Methods in Enzymology (eds C. W Carter & R. M. Sweet). 472–494 (1997)
Abrahams, J. P. & Leslie, A. G. Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr. D 52, 30–42 (1996)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991)
Petrek, M. et al. CAVER: a new tool to explore routes from protein clefts, pockets and cavities. BMC Bioinformatics 7, 316 (2006)
Delano, W. L. The PyMOL molecular graphics system. v.0. 97 〈http://pymol.sourceforge.net/〉 (2002)
Ishitani, R. CueMol; Molecular visualization framework. v.1. 1 〈http://cuemol.sourceforge.jp/〉 (2006)
Acknowledgements
We thank the beam-line staffs at BL41XU of SPring-8 and BL5A of KEK for technical help during data collection, and Y. Sugita and T. Tsukazaki for suggestions. This work was supported by a SORST Program grant from JST (Japan Science and Technology) to O.N., by grants from MEXT to R.I., S.F. and O.N., and by grants from the Society for Research on Umami Taste, the Danone Institute, and the Yamazaki Foundation to O.N.
Author Contributions M.H. designed the research, carried out the crystallization of the full-length MgtE and the structure determinations, and wrote the paper, with editing from S.F., R.I. and O.N. Y.T. carried out the crystallization of the cytosolic domain of MgtE. S.F., R.I. and O.N. assisted with the structural determination. All authors discussed the results and commented on the manuscript. O.N. supervised the work.
The coordinates and structure factors have been deposited in the Protein Data Bank, under the accession codes 2YVX, 2YVY and 2YVZ for the full-length MgtE, and the Mg2+-bound and Mg2+-free cytosolic domains of MgtE, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Discussion, Supplementary Figures S1-S12 with Legends, Supplementary Tables S1-S3 and additional references. (PDF 17697 kb)
Rights and permissions
About this article
Cite this article
Hattori, M., Tanaka, Y., Fukai, S. et al. Crystal structure of the MgtE Mg2+ transporter. Nature 448, 1072–1075 (2007). https://doi.org/10.1038/nature06093
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06093
This article is cited by
-
Structural and functional comparison of magnesium transporters throughout evolution
Cellular and Molecular Life Sciences (2022)
-
Crystal structure of an archaeal CorB magnesium transporter
Nature Communications (2021)
-
Structure determination of the HgcAB complex using metagenome sequence data: insights into microbial mercury methylation
Communications Biology (2020)
-
SLC41A1 is essential for magnesium homeostasis in vivo
Pflügers Archiv - European Journal of Physiology (2019)
-
Metal homeostasis and resistance in bacteria
Nature Reviews Microbiology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.