Abstract
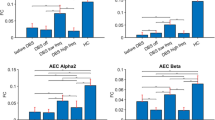
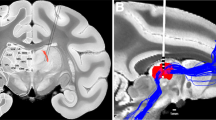
Widespread loss of cerebral connectivity is assumed to underlie the failure of brain mechanisms that support communication and goal-directed behaviour following severe traumatic brain injury. Disorders of consciousness that persist for longer than 12 months after severe traumatic brain injury are generally considered to be immutable; no treatment has been shown to accelerate recovery or improve functional outcome in such cases1,2. Recent studies have shown unexpected preservation of large-scale cerebral networks in patients in the minimally conscious state (MCS)3,4, a condition that is characterized by intermittent evidence of awareness of self or the environment5. These findings indicate that there might be residual functional capacity in some patients that could be supported by therapeutic interventions. We hypothesize that further recovery in some patients in the MCS is limited by chronic underactivation of potentially recruitable large-scale networks. Here, in a 6-month double-blind alternating crossover study, we show that bilateral deep brain electrical stimulation (DBS) of the central thalamus modulates behavioural responsiveness in a patient who remained in MCS for 6 yr following traumatic brain injury before the intervention. The frequency of specific cognitively mediated behaviours (primary outcome measures) and functional limb control and oral feeding (secondary outcome measures) increased during periods in which DBS was on as compared with periods in which it was off. Logistic regression modelling shows a statistical linkage between the observed functional improvements and recent stimulation history. We interpret the DBS effects as compensating for a loss of arousal regulation that is normally controlled by the frontal lobe in the intact brain. These findings provide evidence that DBS can promote significant late functional recovery from severe traumatic brain injury. Our observations, years after the injury occurred, challenge the existing practice of early treatment discontinuation for patients with only inconsistent interactive behaviours and motivate further research to develop therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lombardi, F., Taricco, M., DeTanti, A., Telaro, E. & Liberati, A. Sensory stimulation for brain injured individuals in coma or vegetative state. Cochrane Database Syst. Rev. 2, CD001427 (2002)
Giacino, J. & Whyte, J. The vegetative state and minimally conscious state: current knowledge and remaining questions. J. Head Trauma Rehabil. 20, 30–50 (2005)
Boly, M. et al. Auditory processing in severely brain injured patients: differences between the minimally conscious state and the persistent vegetative state. Arch. Neurol. 61, 233–238 (2004)
Schiff, N. D. et al. fMRI reveals large-scale network activation in minimally conscious patients. Neurology 64, 514–523 (2005)
Giacino, J. T. et al. The minimally conscious state: definition and diagnostic criteria. Neurology 58, 349–353 (2002)
Schiff, N. D. & Purpura, K. P. Towards a neurophysiological basis for cognitive neuromodulation. Thalamus Relat. Syst. 2, 55–69 (2002)
Steriade, M. & Glenn, L. L. Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. J. Neurophysiol. 48, 352–371 (1982)
van der Werf, Y. D., Witter, M. P. & Groenewegen, H. J. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 39, 107–140 (2002)
Wyder, M. T., Massoglia, D. P. & Stanford, T. R. Contextual modulation of central thalamic delay-period activity: representation of visual and saccadic goals. J. Neurophysiol. 91, 2628–2648 (2004)
Kinomura, S., Larssen, J., Gulyas, B. & Roland, P. E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 271, 512–515 (1996)
Paus, T. et al. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J. Cogn. Neurosci. 9, 392–408 (1997)
Giacino, J. T., Kalmar, K. & Whyte, J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 85, 2020–2029 (2004)
Giacino, J. T., Kezmarsky, M. A., DeLuca, J. & Cicerone, K. D. Monitoring rate of recovery to predict outcome in minimally responsive patients. Arch. Phys. Med. Rehabil. 72, 897–901 (1991)
Munkle, M. C., Waldvogel, H. J. & Faull, R. L. The distribution of calbindin, calretinin and parvalbumin immunoreactivity in the human thalamus. J. Chem. Neuroanat. 19, 155–173 (2000)
Jones, E. G. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 24, 595–601 (2001)
Llinas, R. R., Leznik, E. & Urbano, F. J. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc. Natl Acad. Sci. USA 99, 449–454 (2002)
Pfaff, D. Brain Arousal and Information Theory (Harvard Univ. Press, Cambridge, Massachusetts, 2005)
Shirvalkar, P., Seth, M., Schiff, N. D. & Herrera, D. G. Cognitive enhancement through central thalamic deep brain stimulation. Proc. Natl Acad. Sci. USA 103, 17007–17012 (2006)
Schiff, N. D., Hudson, A. E. & Purpura, K. P. Modeling wakeful unresponsiveness: characterization and microstimulation of the central thalamus. Soc. Neurosci. 31st Annual Meeting Abstr. 62.12. (2002)
Morel, A., Liu, J., Wannier, T., Jeanmonod, D. & Rouiller, E. M. Divergence and convergence of thalamocortical projections to premotor and supplementary motor cortex: a multiple tracing study in the macaque monkey. Eur. J. Neurosci. 21, 1007–1029 (2005)
Jennett, B. et al. Neuropathology in vegetative and severely disabled patients after head injury. Neurology 56, 486–490 (2001)
Fins, J. J. Constructing an ethical stereotaxy for severe brain injury: balancing risks, benefits and access. Nature Rev. Neurosci. 4, 323–327 (2003)
Fins, J. J. A proposed ethical framework for interventional cognitive neuroscience: a consideration of deep brain stimulation in impaired consciousness. Neurol. Res. 22, 273–278 (2000)
Acknowledgements
Funding for this study came from the NINDS, Charles A. Dana Foundation, Cleveland Clinic Foundation Brain Neuromodulation Center, Ohio Department of Development BRTT and Third Frontier Program, Jane and Lee Seidman Neuromodulation Research Fund, Cleveland Clinic Innovations, IntElect Medical and the NIDDR. We thank L. Turkstra, E. Montgomery, A. Sharan, D. Vegh and K. Purpura for contributions in planning stages of this work; E. Bagiella for statistical consultation; and C. Allen, D. Pfaff, J. Whyte, M. Gizzi and R. Grewal for expert editorial review.
Author Contributions N.D.S., J.T.G. and A.R.R. contributed equally to the design, implementation and scholarly preparation of this work. N.D.S. and J.T.G. wrote the manuscript. N.D.S., J.T.G. and A.R.R. acted as principal investigators at their performance sites and participated in all phases of the study including evaluation of data and preparation of all parts of the manuscript. A.R.R. and N.D.S. acted as co-principal investigators for the Investigational Device Exemption (Food and Drug Administration, IDE) covering the use of the brain stimulation methods. N.D.S. acted as principal investigator for the initiation of the project and the development grant that formed the basis of the studies. J.T.G. developed, organized and supervised the collection and primary analysis of behavioural data along with K.K. and an independent statistical consultant (E. Bagiella, Columbia University). J.D.V. assisted with the development of the study design, developed the logistic regression models and supervised this data analysis, and assisted in the preparation of manuscript. K.B. analysed the evoked potential response data and prepared the manuscript presentation of the results; K.B. and N.D.S. collected the evoked potential data. M.G., B.F., B.E. and J.O. collected behavioural data and assisted in the development of secondary outcome measures. C.M. served as the patient’s primary care physician and supervised and administered the deep brain electrical stimulation of the patient. Owing to role sequestration, she had no role in data collection or analysis. A.R.R. organized, supervised and carried out the neurosurgical planning, procedures and follow-up, supervised the programming of the neurostimulation and assisted in preparation of the manuscript. J.J.F. developed formal consent procedures and conceptual frameworks with the study primary investigators to address ethical considerations arising in all aspects of the study and preparation of the manuscript. E.J.K., A.M. and K.B. participated in the pre-surgical evaluation, operative and post-operative patient evaluations and neurophysiological evaluations. S.F. provided expert assistance in the design and evaluation of the stimulation protocols. F.P. acted as principal investigator for the planning study for the first year and as a senior advisor throughout the development of the work.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
N.D.S. is an inventor at Cornell University of some of the technology used here. N.D.S., A.M. and A.R.R. are paid consultants and advisors to IntElect Medical Inc., to which the technology has been licensed by Cornell University and in which Cleveland Clinic Foundation and Cornell University have an equity interest. IntElect Medical Inc. provided partial support for the clinical studies reported.
Supplementary information
Supplementary Information
This file contains Supplementary Notes, Supplementary Tables S1-S2, Supplementary Figures 1-7 with Legends, Supplementary Methods; Supplementary Discussion and additional references. The Supplementary Figures illustrate behavioral data from post-operative testing, titration and cross-over phases not included in main text. The Supplementary Notes include narrative clinical history and description of all behavioral metrics and detailed description of logistic regression modeling. The Supplementary Methods include supplementary neurosurgical and titration testing methods. DBS evoked potential studies are also discussed. (PDF 768 kb)
Rights and permissions
About this article
Cite this article
Schiff, N., Giacino, J., Kalmar, K. et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 448, 600–603 (2007). https://doi.org/10.1038/nature06041
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06041
This article is cited by
-
Deep brain stimulation of the central thalamus restores arousal and motivation in a zolpidem-responsive patient with akinetic mutism after severe brain injury
Scientific Reports (2024)
-
Distinct Spectral Profiles of Awake Resting EEG in Disorders of Consciousness: The Role of Frequency and Topography of Oscillations
Brain Topography (2024)
-
Deep brain stimulation in disorders of consciousness: 10 years of a single center experience
Scientific Reports (2023)
-
How deep is the brain? The shallow brain hypothesis
Nature Reviews Neuroscience (2023)
-
The structural connectivity mapping of the intralaminar thalamic nuclei
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.