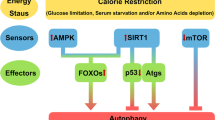
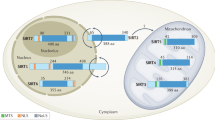
Abstract
Metabolic syndrome threatens health gains made during the past century. Physiological processes degraded by this syndrome are often oppositely affected by calorie restriction, which extends lifespan and prevents disease in rodents. Recent research in the field of ageing has begun to identify important mediators of calorie restriction, offering the hope of new drugs to improve healthspan. Moreover, if metabolic syndrome and calorie restriction are opposite extremes of the same metabolic spectrum, calorie restriction mimetics might provide another therapeutic approach to metabolic syndrome. Sirtuins and other important metabolic pathways that affect calorie restriction may serve as entry points for drugs to treat metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Luchsinger, J. A. A work in progress: the metabolic syndrome. Sci. Aging Knowl. Environ. 10, pe19 (2006).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112, 2735–2752 (2005).
Wilson, P. W. F., D’Agostino, R. B., Parise, H., Sullivan, L. & Meigs, J. B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 112, 3066–3072 (2005).
Weindruch, R. & Walford, R. L. The Retardation of Aging and Disease by Dietary Restriction (Charles C. Thomas, Springfield, Illlinois, 1988).
Holliday, R. Food, reproduction, and longevity: is the extended life span of calorie-restricted animals and evolutionary adaptation? BioEssays 10, 125–127 (1989).
Kaeberlein, M., McVey, M. & Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in saccharomyces cerevisiae by two different machanisms. Genes Dev. 13, 2570–2580 (1999).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in caenorhabditis elegans. Nature 410, 227–230 (2001).
Wood, J. G. et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689 (2004).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Landry, J. et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl Acad. Sci. USA 97, 5807–5811 (2000).
Fernandes, G., Yunis, E.J. & Good, R. A. Suppression of adenocarcinoma by the immunological consequences of calorie restriction. Nature 263, 504–507 (1976).
Zhu, H., Gou, Q. & Mattson, M. P. Dietary restriction protects hippocampal neurons against the death-promoting action of presenilin-1 mutation. Brain Res. 842, 224–229 (1999).
Ingram, D. K., Weindruch, R., Spangler, E. L., Freeman, J. R. & Walford, R. L. Dietary restriction benifits learning and motor performance of aged mice. J. Gerontol. 42, 78–81 (1987).
Sinclair, D. A. & Guarente, L. Extrachromosomal rDNA circles — a cause of aging in yeast. Cell 91, 1033–1042 (1997).
Aguilaniu, H., Gustafsson, L., Rigoulet, M. & Nystrom, T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299, 1751–1753 (2003).
Lin, S. J., Defessez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000).
Lin, S. J. et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344–348 (2002).
Lamming, D. W. et al. HST2 mediates SIR2-independent life-span extension by calorie restriction. Science 309, 1861–1864 (2005).
Lin, S.-J., Ford, E., Haigis, M., Liszt, G. & Guarente, L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 18, 12–16 (2004).
Anderson, R. M., Bitterman, K. J., Wood, J. G., Medvedik, O. & Sinclair, D. A. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharamyces cerevisiae. Nature 423, 181–185 (2003).
Kaeberlein, M., Kirkland, K. T., Fields, S. & Kennedy, B. K. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2, e296 (2004).
Kaeberlein, M. et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1197 (2005).
Rogina, B., Helfand, S. L. & Frankel, S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science 298, 1745 (2002).
Rogina, B. & Helfand, S. L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl Acad. Sci. USA 101, 15998–16003 (2004).
Wang, Y. & Tissenbaum, H. A. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 127, 48–56 (2006).
Chen, D., Steele, A. D., Lindquist, S. & Guarente, L. Increase in activity during calorie restriction requires Sirt1. Science 310, 1641 (2005).
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001).
Vaziri, H. et al. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001).
Motta, M. C. et al. Mammalian SIRT1 represses forkhead transcription factors. Cell 116, 551–563 (2004).
Brunet, A. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 (2004).
Cohen, H. Y. et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls bax-mediated apoptosis. Mol. Cell 13, 627–638 (2004).
Cohen, H. Y. et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305, 390–392 (2004).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429, 771–776 (2004).
Bordone, L. et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biol. 4, e31 (2005).
Moynihan, K. A. et al. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2, 105–117 (2005).
Kitamura, Y. I. et al. FoxO1 protects against pancreatic β cell failure through NeuroD and Mafa induction. Cell Metab. 2, 153–163 (2005).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Nemoto, S., Fergusson, M. M. & Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 280, 16456–16460 (2005).
Accili, D. & Arden, K. C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421–426 (2004).
Taniguchi, C. M., Emanuelli, B. & Kahn, C. R. Critical nodes in signaling pathways: insights into insulin action. Nature Rev. Mol. Cell Biol. 7, 85–96 (2006).
Nisoli, E. et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317 (2005).
Hagopian, K., Ramsey, J. J. & Weindruch, R. Influence of age and calorie restriction on liver glycolytic enxyme activities and metabolite concentrations in mice. Exp. Gerontol. 38, 253–266 (2003).
Wallace, D. C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407 (2005).
Onyango, P., Celic, I., McCaffery, J. M., Boeke, J. D. & Feinberg, A. P. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc. Natl Acad. Sci. USA 99, 13653–13658 (2002).
Schwer, B., North, B. J., Frye, R. A., Ott, M. & Verdin, E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J. Cell Biol. 158, 647–657 (2002).
Haigis, M. C. et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β-cells. Cell 126, 941–956 (2006).
Schwer, B., Bunkenborg, J., Verdin, R. O., Andersen, J. S. & Verdin, E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synsthetase2. Proc. Natl Acad. Sci. USA 103, 10224–10229 (2006).
Hallows, W. C., Lee, S. & Denu, J. M. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl Acad. Sci. USA 103, 10230–10235 (2006).
Starai, V. J., Celic, I., Cole, R. N., Boeke, J. D. & Escalante-Semerena, J. C. Sir2-dependent activationof acetyl-CoA synthetase by deacetylation of active lysine. Science 298, 2390 (2002).
Buckley, B. M. & Williamson, D. H. Origins of blood acetate in the rat. Biochem. J. 166, 539–545 (1977).
Fujino, T., Kondo, J., Ishikawa, M., Morikawa, K. & Yamamoto, T. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J. Biol. Chem. 276, 11420–11426 (2001).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Michishita, E., Park, J. Y., Burneskis, J. M., Barrett, J. C. & Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 16, 4623–4635 (2005).
Ford, E., Voit, R., Liszt, G., Grummt, I. & Guarente, L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 20, 1075–1081 (2006).
Muth, V., Nadaud, S., Grummt, I. & Voit, R. Acetylation of TAFI68, a subunit of TIF-IB/SLI, activates RNA polymerase I transcription. EMBO J. 20, 1353–1362 (2001).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Lin, J., Handschin, C. & Spiegelman, B. M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1, 361–370 (2005).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genet. 34, 267–273 (2003).
Patti, M. E. et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl Acad. Sci. USA 100, 8466–8471 (2003).
Puigserver, P. et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423, 550–555 (2003).
Lin, J. et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell 120, 261–273 (2005).
Wolfrum, C. & Stoffel, M. Coactivation of Foxa2 through Pgc-1β promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 3, 99–110 (2006).
Wolfrum, C., Asilmaz, E., Luca, E., Friedman, J. & Stoffel, M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature 432, 1027–1032 (2004).
Kahn, B. K., Alquier, T., Carling, D. & Hardie, D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1, 15–25 (2005).
Hardie, D. G., Scott, J. W., Pan, D. A. & Hudson, E. R. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 546, 113–120 (2003).
Hawley, S. A. et al. Complexes between the LKB1 tumor suppressor, STRADα/β and Mo25α/β are upstream kinases in the AMP-activated protein kinase cascade. J.Biol. 2, 1–16 (2003).
Woods, A. et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13, 2004–2008 (2003).
Shaw, R. J. et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA 101, 3329–3335 (2004).
Birnbaum, M. J. Activating AMP-activated protein kinase without AMP. Mol. Cell 19, 289–296 (2005).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Brownsey, R. W., Boone, A. N., Elliott, J. E., Kulpa, J. E. & Lee, W. M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 34, 223–227 (2006).
Koo, S.-H. et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1114 (2005).
Canettieri, G. et al. Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling. Cell Metab. 2, 331–338 (2005).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Bauer, J. A. et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1–14 (2006).
Acknowledgements
The author apologizes for the many studies and references that could not be included because of space limitations. Work from the author's laboratory was supported by the NIH.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Leonard Guarente is a founder of Elixir Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Guarente, L. Sirtuins as potential targets for metabolic syndrome. Nature 444, 868–874 (2006). https://doi.org/10.1038/nature05486
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05486
This article is cited by
-
Bioinformatics Analysis of miR-181a and Its Role in Adipogenesis, Obesity, and Lipid Metabolism Through Review of Literature
Molecular Biotechnology (2023)
-
Role of NAD+ and FAD in Ischemic Stroke Pathophysiology: An Epigenetic Nexus and Expanding Therapeutic Repertoire
Cellular and Molecular Neurobiology (2023)
-
Melatonin ameliorates diabetic hyperglycaemia-induced impairment of Leydig cell steroidogenic function through activation of SIRT1 pathway
Reproductive Biology and Endocrinology (2022)
-
Sirtuins are crucial regulators of T cell metabolism and functions
Experimental & Molecular Medicine (2022)
-
SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.