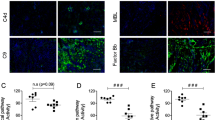
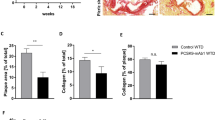
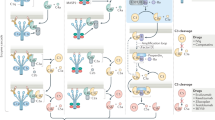
Abstract
Complement-mediated inflammation exacerbates the tissue injury of ischaemic necrosis in heart attacks and strokes, the most common causes of death in developed countries. Large infarct size increases immediate morbidity and mortality and, in survivors of the acute event, larger non-functional scars adversely affect long-term prognosis. There is thus an important unmet medical need for new cardioprotective and neuroprotective treatments. We have previously shown that human C-reactive protein (CRP), the classical acute-phase protein that binds to ligands exposed in damaged tissue and then activates complement1, increases myocardial and cerebral infarct size in rats subjected to coronary or cerebral artery ligation, respectively2,3. Rat CRP does not activate rat complement, whereas human CRP activates both rat and human complement4. Administration of human CRP to rats is thus an excellent model for the actions of endogenous human CRP2,3. Here we report the design, synthesis and efficacy of 1,6-bis(phosphocholine)-hexane as a specific small-molecule inhibitor of CRP. Five molecules of this palindromic compound are bound by two pentameric CRP molecules, crosslinking and occluding the ligand-binding B-face of CRP and blocking its functions. Administration of 1,6-bis(phosphocholine)-hexane to rats undergoing acute myocardial infarction abrogated the increase in infarct size and cardiac dysfunction produced by injection of human CRP. Therapeutic inhibition of CRP is thus a promising new approach to cardioprotection in acute myocardial infarction, and may also provide neuroprotection in stroke. Potential wider applications include other inflammatory, infective and tissue-damaging conditions characterized by increased CRP production, in which binding of CRP to exposed ligands in damaged cells may lead to complement-mediated exacerbation of tissue injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pepys, M. B. & Hirschfield, G. M. C-reactive protein: a critical update. J. Clin. Invest. 111, 1805–1812 (2003)
Griselli, M. et al. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J. Exp. Med. 190, 1733–1739 (1999)
Gill, R., Kemp, J. A., Sabin, C. & Pepys, M. B. Human C-reactive protein increases cerebral infarct size after middle cerebral artery occlusion in adult rats. J. Cereb. Blood Flow Metab. 24, 1214–1218 (2004)
de Beer, F. C. et al. Isolation and characterisation of C-reactive protein and serum amyloid P component in the rat. Immunology 45, 55–70 (1982)
Thompson, D., Pepys, M. B. & Wood, S. P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 7, 169–177 (1999)
Pepys, M. B. et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature 417, 254–259 (2002)
Eda, S., Kaufmann, J., Roos, W. & Pohl, S. Development of a new microparticle-enhanced turbidometric assay for C-reactive protein with superior features in analytical sensitivity and dynamic range. J. Clin. Lab. Anal. 12, 137–144 (1998)
Kushner, I., Broder, M. L. & Karp, D. Control of the acute phase response. Serum C-reactive protein kinetics after acute myocardial infarction. J. Clin. Invest. 61, 235–242 (1978)
de Beer, F. C. et al. Measurement of serum C-reactive protein concentration in myocardial ischaemia and infarction. Br. Heart J. 47, 239–243 (1982)
Taylor, K. E., Giddings, J. C. & van den Berg, C. W. C-reactive protein-induced in vitro endothelial cell activation is an artefact caused by azide and lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 25, 1225–1230 (2005)
Pepys, M. B. CRP or not CRP? That is the question. Arterioscler. Thromb. Vasc. Biol. 25, 1091–1094 (2005)
Pepys, M. B. et al. Pro-inflammatory effects of bacterial recombinant human C-reactive protein are caused by contamination with bacterial products not by C-reactive protein itself. Circ. Res. 97, e97–103 (2005)
Clapp, B. R. et al. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation 111, 1530–1536 (2005)
Hirschfield, G. M. et al. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA 102, 8309–8314 (2005)
Ledue, T. B. et al. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A and mannose-binding protein in human serum. Ann. Clin. Biochem. 35, 745–753 (1998)
Nelson, S. R. et al. Serum amyloid P component in chronic renal failure and dialysis. Clin. Chim. Acta 200, 191–200 (1991)
Bhakdi, S., Torzewski, M., Klouche, M. & Hemmes, M. Complement and atherogenesis. Binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler. Thromb. Vasc. Biol. 19, 2348–2354 (1999)
Hawkins, P. N., Tennent, G. A., Woo, P. & Pepys, M. B. Studies in vivo and in vitro of serum amyloid P component in normals and in a patient with AA amyloidosis. Clin. Exp. Immunol. 84, 308–316 (1991)
Gray, G. A., Mickley, E. J., Webb, D. J. & McEwan, P. E. Localization and function of ET-1 and ET receptors in small arteries post-myocardial infarction: upregulation of smooth muscle ETB receptors that modulate contraction. Br. J. Pharmacol. 130, 1735–1744 (2000)
Miller, A. A., Megson, I. L. & Gray, G. A. Inducible nitric oxide synthase-derived superoxide contributes to hypereactivity in small mesenteric arteries from a rat model of chronic heart failure. Br. J. Pharmacol. 131, 29–36 (2000)
Mora, A. et al. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBO J. 22, 4666–4676 (2003)
Denvir, M. A. et al. Influence of scanning frequency and ultrasonic contrast agent on reproducibility of left ventricular measurements in the mouse. J. Am. Soc. Echocardiogr. 18, 155–162 (2005)
DeLano, W. L. The PyMOL Molecular Graphics System http://www.pymol.org (2002).
Acknowledgements
We thank R. Ganellin for a preliminary synthesis of bis(PC)-H by a different route, D. Moore, M. Neal, M. Singer and R. Stidwell for assistance with rat studies, and P. Vallance for advice. The study was funded by an MRC Programme Grant (M.B.P. and P.N.H.), a project grant from the US NIH National Heart, Lung and Blood Institute's Inflammation and Thrombosis Program (M.B.P.), The Wellcome Trust (S.P.W.) and Pentraxin Therapeutics Ltd. G.M.H. held an MRC Clinical Research Training Fellowship, M.D.S. holds a Royal Society University Research Fellowship and S.V.L. a Novartis Research Fellowship. Author Contributions The study was initiated, designed and directed by M.B.P., and the paper was written and the novel compounds were designed by M.B.P. and S.P.W. In vitro and in vivo experimental work was performed M.B.P., G.M.H., G.A.T., J.R.G., M.C.K and V.B. P.N.H. contributed to study design. Chemical syntheses were designed and performed by S.V.L., R.M.M., M.D.S., A.P. and A.J.A.C., and mass spectrometry was performed by J.A.A. and C.V.R. Myocardial infarction experiments were conducted by G.A.G. and I.S., C.A.S. performed statistical analyses, and S.P.W., M.C.J., S.E.K. and D.T. did the X-ray crystallography.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.B.P. is the inventor on a patent application identifying CRP as a therapeutic target, and M.B.P., S.V.L. and A.J.A.C. are the inventors on a patent application covering CRP inhibitor drugs. These applications are funded by UCL BioMedica PLC, the commercial exploitation arm of UCL, and Pentraxin Therapeutics Ltd, a University College London spinout company of which M.B.P. is a director. The other authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains the Supplementary Methods, Supplementary Table 1 and additional references. (DOC 60 kb)
Rights and permissions
About this article
Cite this article
Pepys, M., Hirschfield, G., Tennent, G. et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 440, 1217–1221 (2006). https://doi.org/10.1038/nature04672
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04672
This article is cited by
-
Regulation of Conformational Changes in C-reactive Protein Alters its Bioactivity
Cell Biochemistry and Biophysics (2022)
-
CRP-Apherese bei akutem Myokardinfarkt bzw. COVID-19
Medizinische Klinik - Intensivmedizin und Notfallmedizin (2022)
-
Extracellular vesicles are associated with C-reactive protein in sepsis
Scientific Reports (2021)
-
Complement in ischaemia–reperfusion injury and transplantation
Seminars in Immunopathology (2021)
-
Income, inflammation and cancer mortality: a study of U.S. National Health and Nutrition Examination Survey mortality follow-up cohorts
BMC Public Health (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.