Abstract
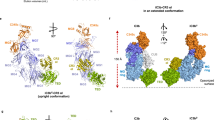
The mammalian complement system is a phylogenetically ancient cascade system that has a major role in innate and adaptive immunity. Activation of component C3 (1,641 residues) is central to the three complement pathways and results in inflammation and elimination of self and non-self targets. Here we present crystal structures of native C3 and its final major proteolytic fragment C3c. The structures reveal thirteen domains, nine of which were unpredicted, and suggest that the proteins of the α2-macroglobulin family evolved from a core of eight homologous domains. A double mechanism prevents hydrolysis of the thioester group, essential for covalent attachment of activated C3 to target surfaces. Marked conformational changes in the α-chain, including movement of a critical interaction site through a ring formed by the domains of the β-chain, indicate an unprecedented, conformation-dependent mechanism of activation, regulation and biological function of C3.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Carroll, M. C. The complement system in regulation of adaptive immunity. Nature Immunol. 5, 981–986 (2004)
Walport, M. J. Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 (2001); Complement. Second of two parts. N. Engl. J. Med. 344, 1140–1144 (2001)
Sunyer, J. O., Zarkadis, I. K. & Lambris, J. D. Complement diversity: a mechanism for generating immune diversity? Immunol. Today 19, 519–523 (1998)
Levashina, E. A. et al. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 104, 709–718 (2001)
Budd, A., Blandin, S., Levashina, E. A. & Gibson, T. J. Bacterial α2-macroglobulins: colonization factors acquired by horizontal gene transfer from the metazoan genome? Genome Biol. 5, R38 (2004)
Bokisch, V. A., Muller-Eberhard, H. J. & Cochrane, C. G. Isolation of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemotactic activity and description of an anaphylatoxin inactivator of human serum. J. Exp. Med. 129, 1109–1130 (1969)
Tack, B. F., Harrison, R. A., Janatova, J., Thomas, M. L. & Prahl, J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc. Natl Acad. Sci. USA 77, 5764–5768 (1980)
Lambris, J. D. The multifunctional role of C3, the third component of complement. Immunol. Today 9, 387–393 (1988)
Law, S. K., Lichtenberg, N. A. & Levine, R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J. Immunol. 123, 1388–1394 (1979)
Muller-Eberhard, H. J. & Gotze, O. C3 proactivator convertase and its mode of action. J. Exp. Med. 135, 1003–1008 (1972)
Fishelson, Z., Pangburn, M. K. & Muller-Eberhard, H. J. Characterization of the initial C3 convertase of the alternative pathway of human complement. J. Immunol. 132, 1430–1434 (1984)
Pangburn, M. K., Schreiber, R. D. & Muller-Eberhard, H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein β1H for cleavage of C3b and C4b in solution. J. Exp. Med. 146, 257–270 (1977)
Ross, G. D., Lambris, J. D., Cain, J. A. & Newman, S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J. Immunol. 129, 2051–2060 (1982)
Seya, T., Turner, J. R. & Atkinson, J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J. Exp. Med. 163, 837–855 (1986)
Harrison, R. A. & Lachmann, P. J. Novel cleavage products of the third component of human complement. Mol. Immunol. 17, 219–228 (1980)
Lachmann, P. J., Pangburn, M. K. & Oldroyd, R. G. Breakdown of C3 after complement activation. Identification of a new fragment C3g, using monoclonal antibodies. J. Exp. Med. 156, 205–216 (1982)
Collaborative Computational Project, Number 4, The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Nagar, B., Jones, R. G., Diefenbach, R. J., Isenman, D. E. & Rini, J. M. X-ray crystal structure of C3d: a C3 fragment and ligand for complement receptor 2. Science 280, 1277–1281 (1998)
Storoni, L. C., McCoy, A. J. & Read, R. J. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D 60, 432–438 (2004)
de Bruijn, M. H. & Fey, G. H. Human complement component C3: cDNA coding sequence and derived primary structure. Proc. Natl Acad. Sci. USA 82, 708–712 (1985)
Huber, R., Scholze, H., Paques, E. P. & Deisenhofer, J. Crystal structure analysis and molecular model of human C3a anaphylatoxin. Hoppe-Seyler's Z. Physiol. Chem. 361, 1389–1399 (1980)
Jenner, L., Husted, L., Thirup, S., Sottrup-Jensen, L. & Nyborg, J. Crystal structure of the receptor-binding domain of α2-macroglobulin. Structure 6, 595–604 (1998)
Thomas, M. L., Janatova, J., Gray, W. R. & Tack, B. F. Third component of human complement: localization of the internal thiolester bond. Proc. Natl Acad. Sci. USA 79, 1054–1058 (1982)
Bramham, J. et al. Functional insights from the structure of the multifunctional C345C domain of C5 of complement. J. Biol. Chem. 280, 10636–10645 (2005)
Taniguchi-Sidle, A. & Isenman, D. E. Interactions of human complement component C3 with factor B and with complement receptors type 1 (CR1, CD35) and type 3 (CR3, CD11b/CD18) involve an acidic sequence at the N-terminus of C3 alpha′-chain. J. Immunol. 153, 5285–5302 (1994)
Sottrup-Jensen, L., Sand, O., Kristensen, L. & Fey, G. H. The α-macroglobulin bait region. Sequence diversity and localization of cleavage sites for proteinases in five mammalian α-macroglobulins. J. Biol. Chem. 264, 15781–15789 (1989)
Lagueux, M., Perrodou, E., Levashina, E. A., Capovilla, M. & Hoffmann, J. A. Constitutive expression of a complement-like protein in toll and JAK gain-of-function mutants of Drosophila.. Proc. Natl Acad. Sci. USA 97, 11427–11432 (2000)
Ishii, N., Wadsworth, W. G., Stern, B. D., Culotti, J. G. & Hedgecock, E. M. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9, 873–881 (1992)
Sahu, A. & Lambris, J. D. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 180, 35–48 (2001)
Pangburn, M. K., Schreiber, R. D. & Muller-Eberhard, H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J. Exp. Med. 154, 856–867 (1981)
Isenman, D. E., Kells, D. I., Cooper, N. R., Muller-Eberhard, H. J. & Pangburn, M. K. Nucleophilic modification of human complement protein C3: correlation of conformational changes with acquisition of C3b-like functional properties. Biochemistry 20, 4458–4467 (1981)
Pangburn, M. K. Spontaneous reformation of the intramolecular thioester in complement protein C3 and low temperature capture of a conformational intermediate capable of reformation. J. Biol. Chem. 267, 8584–8590 (1992)
Law, S. K. & Dodds, A. W. The internal thioester and the covalent binding properties of the complement proteins C3 and C4. Protein Sci. 6, 263–274 (1997)
Gadjeva, M. et al. The covalent binding reaction of complement component C3. J. Immunol. 161, 985–990 (1998)
Dodds, A. W., Ren, X. D., Willis, A. C. & Law, S. K. The reaction mechanism of the internal thioester in the human complement component C4. Nature 379, 177–179 (1996)
van den Elsen, J. M. et al. X-ray crystal structure of the C4d fragment of human complement component C4. J. Mol. Biol. 322, 1103–1115 (2002)
Qazi, U., Gettins, P. G. & Stoops, J. K. On the structural changes of native human α2-macroglobulin upon proteinase entrapment. Three-dimensional structure of the half-transformed molecule. J. Biol. Chem. 273, 8987–8993 (1998)
Daoudaki, M. E., Becherer, J. D. & Lambris, J. D. A 34-amino acid peptide of the third component of complement mediates properdin binding. J. Immunol. 140, 1577–1580 (1988)
Winters, M. S., Spellman, D. S. & Lambris, J. D. Solvent accessibility of native and hydrolyzed human complement protein 3 analyzed by hydrogen/deuterium exchange and mass spectrometry. J. Immunol. 174, 3469–3474 (2005)
Oran, A. E. & Isenman, D. E. Identification of residues within the 727–767 segment of human complement component C3 important for its interaction with factor H and with complement receptor 1 (CR1, CD35). J. Biol. Chem. 274, 5120–5130 (1999)
Lambris, J. D. et al. Dissection of CR1, factor H, membrane cofactor protein, and factor B binding and functional sites in the third complement component. J. Immunol. 156, 4821–4832 (1996)
Hammer, C. H., Wirtz, G. H., Renfer, L., Gresham, H. D. & Tack, B. F. Large scale isolation of functionally active components of the human complement system. J. Biol. Chem. 256, 3995–4006 (1981)
Otwinowski, Z. & Minor, W. Processing X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993)
Terwilliger, T. C. & Berendzen, J. Automated MAD and MIR structure solution. Acta Crystallogr. D 55, 849–861 (1999)
Kleywegt, G. J. & Jones, T. A. Software for handling macromolecular envelopes. Acta Crystallogr. D 55, 941–944 (1999)
Perrakis, A., Morris, R. & Lamzin, V. S. Automated protein model building combined with iterative structure refinement. Nature Struct. Biol. 6, 458–463 (1999)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Brunger, A. T. et al. Crystallography NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Solomon, K. R., Sharma, P., Chan, M., Morrison, P. T. & Finberg, R. W. CD109 represents a novel branch of the α2-macroglobulin/complement gene family. Gene 327, 171–183 (2004)
Acknowledgements
We acknowledge the help of beamline scientists of the EMBL/ESRF and in particular R. B. G. Ravelli for help in data collection, and A. Perrakis and D. Egan for help and use of their crystallization robots. We thank A. T. Brunger, M. Bowen, B. DeLaBarre, J. D. Lambris, C. W. Vogel, D. Fritzinger, T. Springer and T. K. Sixma for critically reading the manuscript. This work was supported by a ‘Pionier’ programme grant (P.G.) of the Council for Chemical Sciences of the Netherlands Organization for Scientific Research (NWO-CW), the Dutch Kidney Foundation (A.R.), the Swedish Research Council (B.N.) and faculty grants at the University of Kalmar (K.N.-E.). A.R. does not support the evolutionary implications.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Co-ordinates and structure factors have been deposited in the Protein Data Bank under accession numbers 2A73 (C3) and 2A74 (C3c). Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure Legends
Figure captions for Supplementary Tables S1 and S2 and Supplementary Figures S1 to S6. (DOC 115 kb)
Supplementary Table S1
Diffraction data, structure solution and refinement statistics for C3c and C3. (PDF 51 kb)
Supplementary Table S2
Domain rotation and translation between C3 and C3c. (PDF 34 kb)
Supplementary Figure S1
Electron density of the α′NT region in C3c and the thioester in C3. (PDF 776 kb)
Supplementary Figure S2
Stereo representations of C3 and C3c. Secondary structure assignments and alignment of C3 with α2M family members. Structure-based sequence alignment of the eight MG domains of C3. Superposition of the eight MG domains of C3. (PDF 1190 kb)
Supplementary Figure S3
Structures of individual domains of C3 and C3c superposed. (PDF 1968 kb)
Supplementary Figure S4
Electrostatic surface potential of C3 and C3c. (PDF 217 kb)
Supplementary Figure S5
Sequence conservation of the β bridge, 205YVLP208, and the hinge 745FPES748. (PDF 387 kb)
Supplementary Figure S6
Conglutinin binding site and factor I cleavage sites in the CUB domain. (PDF 813 kb)
Rights and permissions
About this article
Cite this article
Janssen, B., Huizinga, E., Raaijmakers, H. et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature 437, 505–511 (2005). https://doi.org/10.1038/nature04005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04005
This article is cited by
-
Current Understanding of Complement Proteins as Therapeutic Targets for the Treatment of Immunoglobulin A Nephropathy
Drugs (2023)
-
Integrated glycomics and genetics analyses reveal a potential role for N-glycosylation of plasma proteins and IgGs, as well as the complement system, in the development of type 1 diabetes
Diabetologia (2023)
-
Insight into mode-of-action and structural determinants of the compstatin family of clinical complement inhibitors
Nature Communications (2022)
-
Invariant surface glycoprotein 65 of Trypanosoma brucei is a complement C3 receptor
Nature Communications (2022)
-
The crystal structure of iC3b-CR3 αI reveals a modular recognition of the main opsonin iC3b by the CR3 integrin receptor
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.