Abstract
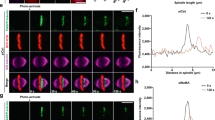
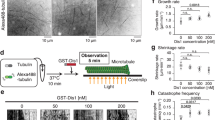
Microtubules of the mitotic spindle form the structural basis for chromosome segregation. In metaphase, microtubules show high dynamic instability, which is thought to aid the ‘search and capture’ of chromosomes for bipolar alignment on the spindle. Microtubules suddenly become more stable at the onset of anaphase, but how this change in microtubule behaviour is regulated and how important it is for the ensuing chromosome segregation are unknown1,2,3,4. Here we show that in the budding yeast Saccharomyces cerevisiae, activation of the phosphatase Cdc14 at anaphase onset is both necessary and sufficient for silencing microtubule dynamics. Cdc14 is activated by separase, the protease that triggers sister chromatid separation, linking the onset of anaphase to microtubule stabilization5,6. If sister chromatids separate in the absence of Cdc14 activity, microtubules maintain high dynamic instability; this correlates with defects in both the movement of chromosomes to the spindle poles (anaphase A) and the elongation of the anaphase spindle (anaphase B). Cdc14 promotes localization of microtubule-stabilizing proteins to the anaphase spindle, and dephosphorylation of the kinetochore component Ask1 contributes to both the silencing of microtubule turnover and successful anaphase A.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others

References
Zhai, Y., Kronebusch, P. J. & Borisy, G. G. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J. Cell Biol. 131, 721–734 (1995)
Mallavarapu, A., Sawin, K. & Mitchison, T. A switch in microtubule dynamics at the onset of anaphase B in the mitotic spindle of Schizosaccaromyces pombe. Curr. Biol. 9, 1423–1426 (1999)
Maddox, P. S., Bloom, K. S. & Salmon, E. D. The polarity and dynamics of microtubule assembly in the budding yeast Saccharomyces cerevisiae. Nature Cell Biol. 2, 36–41 (2000)
Kline-Smith, S. L. & Walczak, C. E. Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol. Cell 15, 317–327 (2004)
Stegmeier, F., Visintin, R. & Amon, A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108, 207–220 (2002)
Sullivan, M. & Uhlmann, F. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nature Cell Biol. 5, 249–254 (2003)
Belmont, L. D., Hyman, A. A., Sawin, K. E. & Mitchison, T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell 62, 579–589 (1990)
Verde, F., Labbé, J.-C., Dorée, M. & Karsenti, E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature 343, 233–238 (1990)
Uhlmann, F., Wernic, D., Poupart, M.-A., Koonin, E. V. & Nasmyth, K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103, 375–386 (2000)
Straight, A. F., Marshall, W. F., Sedat, J. W. & Murray, A. W. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science 277, 574–578 (1997)
Goshima, G. & Yanagida, M. Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell 100, 619–633 (2000)
Tanaka, T., Fuchs, J., Loidl, J. & Nasmyth, K. Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nature Cell Biol. 2, 492–499 (2000)
Winey, M. et al. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol. 129, 1601–1615 (1995)
Zeng, X. et al. Slk19p is a centromere protein that functions to stabilize mitotic spindles. J. Cell Biol. 146, 415–425 (1999)
Miller, R. K. et al. The kinesin-related proteins, Kip2p and Kip3p, function differently in nuclear migration in yeast. Mol. Biol. Cell 9, 2051–2068 (1998)
Pereira, G. & Schiebel, E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science 302, 2120–2124 (2003)
Sullivan, M., Higuchi, T., Katis, V. L. & Uhlmann, F. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell 117, 471–482 (2004)
Sullivan, M., Hornig, N. C. D., Porstmann, T. & Uhlmann, F. Studies on substrate recognition by the budding yeast separase. J. Biol. Chem. 279, 1191–1196 (2004)
Li, Y. & Elledge, S. J. The DASH complex component Ask1 is a cell cycle-regulated Cdk substrate in Saccharomyces cerevisiae. Cell Cycle 2, 143–148 (2003)
Kosco, K. A. et al. Control of microtubule dynamics by Stu2p is essential for spindle orientation and metaphase chromosome alignment in yeast. Mol. Biol. Cell 12, 2870–2880 (2001)
van Breugel, M., Drechsel, D. & Hyman, A. Stu2p, the budding yeast member of the conserved Dis1/XMAP215 family of microtubule-associated proteins is a plus end-binding microtubule destabilizer. J. Cell Biol. 161, 359–369 (2003)
Yin, H., You, L., Pasqualone, D., Kopski, K. M. & Huffaker, T. C. Stu1p is physically associated with β-tubulin and is required for structural integrity of the mitotic spindle. Mol. Biol. Cell 13, 1881–1892 (2002)
Maiato, H. et al. Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell 113, 891–904 (2003)
Mishima, M., Pavicic, V., Grüneberg, U., Nigg, E. A. & Glotzer, M. Cell cycle regulation of central spindle assembly. Nature 430, 908–913 (2004)
Straight, A. F., Sedat, J. W. & Murray, A. W. Time-lapse microscopy reveals unique roles for kinesins during anaphase in budding yeast. J. Cell Biol. 143, 687–694 (1998)
Surana, U. et al. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 12, 1969–1978 (1993)
Murray, A. W., Desai, A. B. & Salmon, E. D. Real time observation of anaphase in vitro. Proc. Natl Acad. Sci. USA 93, 12327–12332 (1996)
Wheatley, S. P. et al. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J. Cell Biol. 138, 385–393 (1997)
Parry, D. H., Hickson, G. R. X. & O'Farrell, P. H. Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr. Biol. 13, 647–653 (2003)
Tournebize, R. et al. Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J. 16, 5537–5549 (1997)
Acknowledgements
We thank S. Elledge, J. Kilmartin and A. Straight for reagents, R. Carazo-Salas and A. Nicol for advice on microscopy, J. Cau, T. Davis, A. Hyman, T. Toda and all members of our laboratory for helpful discussions and critical reading of the manuscript, and in particular M. Sullivan for help at the outset of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure S1
Summary of microtubule dynamics measurements. (PDF 389 kb)
Supplementary Figure S2
Cdc14 rescues spindle stability during TEV protease-triggered anaphase. (PDF 795 kb)
Supplementary Figure S3
Ectopic Cdc14 in metaphase reduces centromere oscillations. (PDF 182 kb)
Supplementary Figure S4
Rescue of spindle stability in the absence of Cdc14 by deletion of Kip3. (PDF 187 kb)
Supplementary Movie 1
Spindle elongation during separase-triggered anaphase. (MPG 339 kb)
Supplementary Movie 2
Spindle elongation during TEV protease-triggered anaphase, example 1. (MPG 251 kb)
Supplementary Movie 3
Spindle elongation during TEV protease-triggered anaphase, example 2 (MPG 207 kb)
Rights and permissions
About this article
Cite this article
Higuchi, T., Uhlmann, F. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature 433, 171–176 (2005). https://doi.org/10.1038/nature03240
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03240
This article is cited by
-
Cell-cycle phospho-regulation of the kinetochore
Current Genetics (2021)
-
Prognostic significance of TOP2A in non-small cell lung cancer revealed by bioinformatic analysis
Cancer Cell International (2019)
-
A PxL motif promotes timely cell cycle substrate dephosphorylation by the Cdc14 phosphatase
Nature Structural & Molecular Biology (2018)
-
Regulation of kinetochore configuration during mitosis
Current Genetics (2018)
-
FEAR-mediated activation of Cdc14 is the limiting step for spindle elongation and anaphase progression
Nature Cell Biology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.