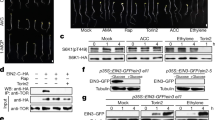
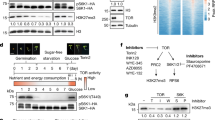
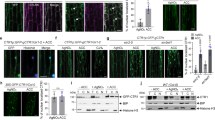
Abstract
Glucose is a global regulator of growth and metabolism that is evolutionarily conserved from unicellular microorganisms to multicellular animals and plants1. In photosynthetic plants, glucose shows hormone-like activities and modulates many essential processes, including embryogenesis, germination, seedling development, vegetative growth, reproduction and senescence2,3. Genetic and phenotypic analyses of Arabidopsis mutants with glucose-insensitive (gin) and glucose-oversensitive (glo) phenotypes have identified an unexpected antagonistic interaction between glucose and the plant stress hormone ethylene. The ethylene-insensitive etr1 and ein2 mutants have glo phenotypes, whereas the constitutive ethylene signalling mutant ctr1 is allelic to gin4 (refs 4, 5). The precise molecular mechanisms underlying the complex signalling network that governs plant growth and development in response to nutrients and plant hormones are mostly unknown. Here we show that glucose enhances the degradation of ETHYLENE-INSENSITIVE3 (EIN3), a key transcriptional regulator in ethylene signalling6,7, through the plant glucose sensor hexokinase8. Ethylene, by contrast, enhances the stability of EIN3. The ein3 mutant has a glo phenotype, and overexpression of EIN3 in transgenic Arabidopsis decreases glucose sensitivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rolland, F., Winderickx, J. & Thevelein, J. M. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem. Sci. 26, 310–317 (2001)
Smeekens, S. Sugar-induced signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 49–81 (2000)
Rolland, F., Moore, B. & Sheen, J. Sugar sensing and signaling in plants. Plant Cell 14, S185–S205 (2002)
Zhou, L., Jang, J.-C., Jones, T. L. & Sheen, J. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc. Natl Acad. Sci. USA 95, 10294–10299 (1998)
Cheng, W.-H. et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and function. Plant Cell 14, 2723–2743 (2002)
Chao, Q. et al. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSTIVE3 and related proteins. Cell 89, 1133–1144 (1997)
Solano, R., Stepanova, A., Chao, Q. & Ecker, J. R. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSTIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 12, 3703–3714 (1998)
Moore, B. et al. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light and hormonal signaling. Science 300, 332–336 (2003)
Chang, C., Kwok, S. F., Bleecker, A. B. & Meyerowitz, E. M. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262, 539–544 (1993)
Alonso, J. M. et al. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152 (1999)
Jang, J.-C. & Sheen, J. Sugar sensing in higher plants. Plant Cell 6, 1665–1679 (1994)
Zuo, J., Niu, Q.-W. & Chua, N.-H. An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24, 265–273 (2000)
Sheen, J. Protein phosphatase activity is required for light-inducible gene expression in maize. EMBO J. 12, 3497–3505 (1993)
Yanagisawa, S. & Sheen, J. Involvement of maize DOF zinc finger proteins in tissue-specific and light-regulated gene expression. Plant Cell 10, 75–89 (1998)
Hwang, I. & Sheen, J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413, 383–389 (2001)
Kosugi, S. & Ohashi, Y. Cloning and DNA-binding properties of a tobacco ethylene-insensive3 (EIN3) homolog. Nucleic Acids Res. 28, 960–967 (2000)
Chang, C.-C. et al. Functional analysis of two maize cDNAs encoding T7-like RNA polymerases. Plant Cell 11, 911–926 (1999)
Wang, K. L.-C., Li, H. & Ecker, J. R. Ethylene biosynthesis and signaling networks. Plant Cell 14, S131–S151 (2002)
Naidoo, N., Song, W., Hunter-Ensor, M. & Sehgal, A. A role for proteasome in the light response of the timeless clock protein. Science 285, 1737–1741 (1999)
Osterlund, M. T. et al. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462–466 (2000)
Ravid, T. et al. The ubiquitin–proteasome pathway mediates the regulated degradation of mammalian 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 275, 35840–35847 (2000)
Fujimoto, S. Y., Ohta, M., Usui, A., Shinshi, H. & Ohme-Takagi, M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12, 393–404 (2000)
Kovtun, Y., Chiu, W.-L., Zeng, W. & Sheen, J. Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature 395, 716–720 (1998)
Kieber, J. J., Rothenberg, M., Gregg, R., Feldmann, K. A. & Ecker, J. R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72, 427–441 (1993)
Gray, W. M., Kepinski, S., Rouse, D. & Estelle, M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414, 271–276 (2001)
Lopez-Molina, L., Mongrand, S. & Chua, N.-H. A postgermination development arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc. Natl Acad. Sci. USA 98, 4782–4787 (2001)
Silverstone, A. L. et al. Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13, 1555–1566 (2001)
He, J.-X. et al. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl Acad. Sci. USA 99, 10185–10190 (2002)
Vierstra, R. D. The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends Plant Sci. 8, 135–142 (2003)
Sheen, J.-Y. & Bogorad, L. Expression of ribulose bisphosphate carboxylase large subunit and three small subunit genes in two cell types of maize leaves. EMBO J. 13, 3417–3422 (1986)
Acknowledgements
We thank B. Lam for help with antibody preparation and for providing plants; T. Miwa for help with generating transgenic Arabidopsis; M. V. Parthasarathy for the actin antibody; F. Rolland and B. Seed for critically reading the manuscript; J. Callis for the UBQ10–GUS construct; B. Moore for the AtXHK1 mutant; Y. Niwa for the TP–GFP construct; and Y.-H. Cho and R. Patharkar for advice. The work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to S.Y.), and the National Science Foundation and the National Institutes of Health (to J.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Yanagisawa, S., Yoo, SD. & Sheen, J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425, 521–525 (2003). https://doi.org/10.1038/nature01984
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01984
This article is cited by
-
Comparative analysis of drought responsive transcriptome in Brassica napus genotypes with contrasting drought tolerance under different potassium levels
Euphytica (2023)
-
Carbohydrate regulation response to cold during rhizome bud dormancy release in Polygonatum kingianum
BMC Plant Biology (2022)
-
Sugar metabolism during pre- and post-fertilization events in plants under high temperature stress
Plant Cell Reports (2022)
-
Interaction between fructan metabolism and plant growth regulators
Planta (2022)
-
Enhanced NRT1.1/NPF6.3 expression in shoots improves growth under nitrogen deficiency stress in Arabidopsis
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.