Abstract
The disease anthrax is caused by lethal factor1, an enzyme component of the toxin produced by the spore-forming bacterium Bacillus anthracis2. Here we describe substrate molecules for this factor that offer a means for high-throughput screening of potential inhibitors for use in anthrax treatment3. Our assay should help to answer the urgent call for new and specific therapies4 to combat this pathogen after its recent emergence as a terrorist bioweapon.
Similar content being viewed by others
Main
The clinical presentation and outcome of anthrax in humans depend on the route of entry. Cutaneous anthrax is rarely fatal, whereas systemic anthrax, which follows inhalation of bacterial spores, is more serious. In addition to the metalloprotease lethal factor (LF), the anthrax toxin contains protective antigen, which mediates the entry of LF into macrophages and other cells1,5.
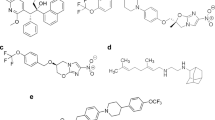
Lethal factor is a proteolytic enzyme that specifically cleaves signalling proteins of the MAPK-kinase family at their amino termini6,7,8,9, targeting a consensus sequence motif8 (see supplementary information). We have used this sequence to create p-anilide peptide substrates for lethal factor metalloprotease that have favourable kinetic characteristics (see supplementary information). A fluorescent coumarin derivative was also effective as a lethal factor substrate, enabling the activity of minute amounts of lethal factor (5% or less of that detectable with p-nitroanilide) to be detected.
With these substrates, lethal factor metalloproteolytic activity can be assayed on plate readers with visible-light or fluorescence detectors (available in most hospital and research laboratories); 1–2 nanograms of lethal factor can be detected in about 200 μl buffer. Our synthetic substrates should be useful for high-throughput screening of chemical libraries to identify specific inhibitors of lethal factor metalloproteolytic activity.
Conversion of the peptide substrates of metalloproteases into hydroxylamine derivatives generates competitive inhibitors of these enzymes10. We therefore investigated the inhibition of lethal factor by hydroxyl-amine derivatives of our peptide substrates in vitro, and found that these hydroxamates give nanomolar inhibition constants; the derivative In-2-LF, with a Ki of 1 nM, is the most powerful of these inhibitors.
As anthrax lethal factor acts in the cell cytosol2,4, potential inhibitors must be able to enter cells to be effective. In-1-LF and In-2-LF both include a strongly basic sequence of amino acids with sequences that resemble those of peptides that cross the plasma membrane11. We found that these two peptides inhibit lethal factor's cytotoxicity in the macrophage cell lines RAW264.7 and J774.A1, which are commonly used to assay lethal factor, with In-2-LF again being the more effective (Fig. 1a, b). In-2-LF also inhibits cleavage of MEK-3 (used here as a paradigm of MAPK-kinase cleavage in general; Fig. 1c). Further evidence of the inhibitor's entry into these cells was obtained by using a fluorescein derivative of In-1-LF (results not shown).
a, Transmission light micrographs of control cells (left) and of cells treated with anthrax toxin (LeTx, consisting of 200 ng ml−1 LF and 400 ng ml−1 protective antigen (PA)) in the absence (middle) or presence (right) of 40 µM In-2-LF for 1 h. LeTx at these concentrations kills cells (middle), whereas In-2-LF reduces cell death (right). b, Protection of RAW264.7 macrophages by In-2-LF. Cells were plated in 96-well plates at a density of 2 × 104 cells per well in DMEM medium supplemented with heat-inactivated fetal calf serum, 1 d before treatment with LeTx (100 ng ml−1 LF and 400 ng ml−1 PA in serum-free medium); the indicated concentrations of In-2-LF peptide were added 5 min after LeTx. After 2 h, cells were washed with PBS and cell death was assayed with MTS tetrazolium (Promega; control was treated with LeTx only). Results are averages from three independent experiments. c, Cleavage of MEK-3b in RAW264.7 cells treated with 800 ng ml−1 LeTx and the indicated concentrations of In-2-LF, as determined by western blotting with anti-MEK3 antibodies (Santa Cruz). Upper and lower bands in each panel are the uncleaved and LF-cleaved forms of MEK-3b, respectively; after 1 or 2 hours of incubation, In-2-LF inhibits the LF-mediated conversion of the uncleaved to the cleaved form in a dose-dependent way (as evaluated by the band ratio), but after 4 hours most MEK-3b has undergone cleavage because In-2-LF acts by competitive inhibition.
References
Hanna, P. Curr. Topics Microbiol. Immunol. 225, 13–35 (1998).
Mock, M. & Fouet, A. Annu. Rev. Microbiol. 55, 647–671 (2001).
Chaudry, G. J., Moayeri, M., Liu, S. & Leppla, S. H. Trends Microbiol. 10, 58–62 (2002).
Friedlander, A. M. Nature 414, 160–161 (2001).
Friedlander, A. M. J. Biol. Chem. 261, 7123–7126 (1986).
Duesbery, N. S. et al. Science 280, 734–737 (1998).
Vitale, G. et al. Biochem. Biophys. Res. Commun. 248, 706–711 (1998).
Vitale, G., Bernardi, L., Napolitani, G., Mock, M. & Montecucco, C. Biochem. J. 352, 739–745 (2000).
Pannifer, A. D. et al. Nature 414, 229–233 (2001).
Roques, B. P. Trends Pharmacol. Sci. 21, 475–483 (2000).
Schwarze, S. R., Hruska, K. A. & Dowdy, S. F. Trends Cell Biol. 10, 290–295 (2000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
brief communications is intended to provide a forum for both brief, topical reports of general scientific interest and technical discussion of recently published material of particular interest to non-specialist readers. Priority will be given to contributions that have fewer than 500 words, 10 references and only one figure. Detailed guidelines are available on Nature's website (http://www.nature.com/nature) or on request from nature@nature.com
Supplementary information
Rights and permissions
About this article
Cite this article
Tonello, F., Seveso, M., Marin, O. et al. Screening inhibitors of anthrax lethal factor. Nature 418, 386 (2002). https://doi.org/10.1038/418386a
Issue Date:
DOI: https://doi.org/10.1038/418386a
This article is cited by
-
Effects of metalloprotease anthrax lethal factor on its peptide-based inhibitor R9LF-1
Molecular and Cellular Biochemistry (2015)
-
Structure-based pharmacophore modeling and virtual screening to identify novel inhibitors for anthrax lethal factor
Medicinal Chemistry Research (2014)
-
Inhibition of anthrax lethal factor: lability of hydroxamate as a chelating group
Applied Microbiology and Biotechnology (2012)
-
Quantum mechanical/molecular mechanical study of anthrax lethal factor catalysis
Theoretical Chemistry Accounts (2011)
-
The biology and future prospects of antivirulence therapies
Nature Reviews Microbiology (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.