Abstract
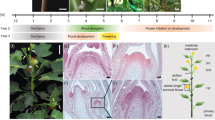
The transition from vegetative to reproductive growth is an essential process in the life cycle of plants. Plant floral induction pathways respond to both environmental and endogenous cues and much has been learnt about these genetic pathways by studying mutants of Arabidopsis1,2. Gibberellins (GAs) are plant growth regulators important in many aspects of plant growth and in Arabidopsis they promote flowering3,4,5. Here we provide genetic evidence that GAs inhibit flowering in grapevine. A grapevine dwarf mutant derived from the L1 cell layer of the champagne cultivar Pinot Meunier produces inflorescences along the length of the shoot where tendrils are normally formed. The mutated gene associated with the phenotype is a homologue of the wheat ‘green revolution’ gene Reduced height-1 (ref. 6) and the Arabidopsis gene GA insensitive (GAI)7. The conversion of tendrils to inflorescences in the mutant demonstrates that the grapevine tendril is a modified inflorescence inhibited from completing floral development by GAs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hempel, F. D., Welch, D. R. & Feldman, L. J. Floral induction and determination: where is flowering controlled? Trends Plant Sci. 5, 17–21 (2000).
Araki, T. Transition from vegetative to reproductive phase. Curr. Opin. Plant Biol. 4, 63–68 (2001).
Blazquez, M. A., Green, R., Nilsson, O., Sussman, M. R. & Weigel, D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell 10, 791–800 (1998).
Blazquez, M. A. & Weigel, D. Integration of floral inductive signals in Arabidopsis. Nature 404, 889–892 (2000).
Langridge, J. Effect of day-length and gibberellic acid on the flowering of Arabidopsis. Nature 180, 36–37 (1957).
Peng, J. et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400, 256–261 (1999).
Peng, J. et al. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 11, 3194–3205 (1997).
Rives, M. Vigour, pruning, cropping in the grapevine (Vitis vinifera L.). I. A literature review. Agronomie 20, 79–91 (2000).
Viala, P. & Vermorel, V. Ampélographie, Tome II (Masson, Paris, 1901).
Skene, K. G. M. & Barlass, M. Studies on the fragmented shoot apex of grapevine. IV. Separation of phenotypes in a periclinal chimera in vitro. J. Exp. Bot. 34, 1271–1280 (1983).
Thompson, M. M. & Olmo, H. P. Cytohistological studies of cytochimeric and tetraploid grapes. Am. J. Bot. 50, 901–906 (1963).
Franks, T., Botta, R. & Thomas, M. R. Chimerism in grapevines: implications for cultivar identity, ancestry and genetic improvement. Theor. Appl. Genet. 104, 192–199 (2002).
Pratt, C. Reproductive anatomy in cultivated grapes: a review. Am. J. Enol. Vitic. 22, 92–109 (1971).
Mullins, M. G. Regulation of inflorescence growth in cuttings of the grapevine (Vitis vinifera L.). J. Exp. Bot. 19, 532–543 (1968).
Utsunomiya, N., Sugiura, A. & Tomana, T. Effect of CCC and pinching on inflorescence induction and development on lateral shoots in grapevine. J. Jpn Soc. Hort. Sci. 47, 151–157 (1978).
Boss, P. K. & Thomas, M. R. Tendrils, inflorescences and fruitfulness: A molecular perspective. Aust. J. Grape Wine Res. 6, 168–174 (2000).
Koornneef, M. et al. A gibberellin insensitve mutant of Arabidopsis thaliana. Physiol. Plant. 65, 33–39 (1985).
Peng, J. et al. Extragenic suppressors of the Arabidopsis gai mutation alter the dose–response relationship of diverse gibberellin responses. Plant Physiol. 119, 1199–1207 (1999).
Pysh, L. D., Wysocka-Diller, J. W., Camilleri, C., Bouchez, D. & Benfey, P. N. The GRAS gene family in Arabidopsis: Sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 18, 111–119 (1999).
Neff, M. M., Neff, J. D., Chory, J. & Pepper, A. E. dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: Experimental applications in Arabidopsis thaliana genetics. Plant J. 14, 387–392 (1998).
Ellis, R. H., Hong, T. D. & Roberts, E. H. A note on the development of a practical procedure for promoting the germination of dormant seed of grape (Vitis spp.). Vitis 22, 211–219 (1983).
Peng, J. & Harberd, N. P. Transposon-associated somatic gai-loss sectors in Arabidopsis. Plant Sci. 130, 181–188 (1997).
Simpson, G. G., Gendall, A. R. & Dean, C. When to switch to flowering. Annu. Rev. Cell Dev. Biol. 15, 519–550 (1999).
Buttrose, M. S. Climatic factors and fruitfulness in grapevines. Hort. Abstr. 44, 319–326 (1974).
Boss, P. K., Davies, C. & Robinson, S. P. Analysis of the expression of anthocyanin pathway genes in developing Vitis vinifera L. cv. Shiraz grape berries and the implications for pathway regulation. Plant Physiol. 111, 1059–1066 (1996).
Frohman, M. A., Dush, M. K. & Martin, G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc. Natl Acad. Sci. USA 85, 8998–9002 (1988).
Thomas, M. R., Matsumoto, S., Cain, P. & Scott, N. S. Repetitive DNA of grapevine: classes present and sequences suitable for cultivar identification. Theor. Appl. Genet. 86, 173–180 (1993).
Acknowledgements
We thank R. King and A. Poole for the GA analyses by GC–MS; T. Franks for plant material; S. McClure for help with the scanning electron mictroscopy; and D. MacKenzie and C. Reich for technical assistance. This work was supported in part by the Grape and Wine Research and Development Corporation, Dried Fruits Research and Development Council and the Cooperative Research Centre for Viticulture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests
Supplementary information
Rights and permissions
About this article
Cite this article
Boss, P., Thomas, M. Association of dwarfism and floral induction with a grape ‘green revolution’ mutation. Nature 416, 847–850 (2002). https://doi.org/10.1038/416847a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/416847a
This article is cited by
-
Near-gapless and haplotype-resolved apple genomes provide insights into the genetic basis of rootstock-induced dwarfing
Nature Genetics (2024)
-
Chimeras in Merlot grapevine revealed by phased assembly
BMC Genomics (2023)
-
Genome-wide Transcriptome Analysis Reveals the Gene Regulatory Network in Star Fruit Flower Blooming
Tropical Plant Biology (2023)
-
Fine Mapping Identifies ClTFL1 Encodes a TERMINAL FLOWER 1 Protein as Putative Candidate Gene for Inflorescence Architecture and Tendril Development and in Watermelon
Journal of Plant Growth Regulation (2023)
-
Auxin regulation on crop: from mechanisms to opportunities in soybean breeding
Molecular Breeding (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.