Abstract
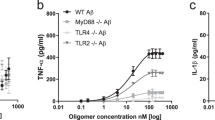
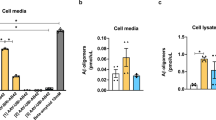
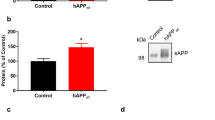
Although extensive data support a central pathogenic role for amyloid β protein (Aβ) in Alzheimer's disease1, the amyloid hypothesis remains controversial, in part because a specific neurotoxic species of Aβ and the nature of its effects on synaptic function have not been defined in vivo. Here we report that natural oligomers of human Aβ are formed soon after generation of the peptide within specific intracellular vesicles and are subsequently secreted from the cell. Cerebral microinjection of cell medium containing these oligomers and abundant Aβ monomers but no amyloid fibrils markedly inhibited hippocampal long-term potentiation (LTP) in rats in vivo. Immunodepletion from the medium of all Aβ species completely abrogated this effect. Pretreatment of the medium with insulin-degrading enzyme, which degrades Aβ monomers but not oligomers, did not prevent the inhibition of LTP. Therefore, Aβ oligomers, in the absence of monomers and amyloid fibrils, disrupted synaptic plasticity in vivo at concentrations found in human brain and cerebrospinal fluid. Finally, treatment of cells with γ-secretase inhibitors prevented oligomer formation at doses that allowed appreciable monomer production, and such medium no longer disrupted LTP, indicating that synaptotoxic Aβ oligomers can be targeted therapeutically.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Selkoe, D. J. Alzheimer's disease: genes, proteins and therapies. Physiol. Rev. 81, 742–761 (2001).
Pike, C. J. Walencewicz, A. J., Glabe, C. G. & Cotman, C. W. In vitro aging of β-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 563, 311–314 (1991).
Lorenzo, A. & Yankner, B. A. β-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc. Natl Acad. Sci. USA 91, 12243–12247 (1994).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
Dickson, D. W. et al. Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol. Aging 16, 285–298 (1995).
Lue, L. F. et al. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am. J. Pathol. 155, 853–862 (1999).
McLean, C. A. et al. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann. Neurol. 46, 860–866 (1999).
Podlisny, M. B. et al. Aggregation of secreted amyloid β-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J. Biol. Chem. 270, 9564–9570 (1995).
Morishima-Kawashima, M. & Ihara, Y. The presence of amyloid β-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry 37, 15247–15253 (1998).
Walsh, D. M., Tseng, B. P., Rydel, R. E., Podlisny, M. B. & Selkoe, D. J. Detection of intracellular oligomers of amyloid β-protein in cells derived from human brain. Biochemistry 39, 10831–10839 (2000).
Xia, W. M. et al. Enhanced production and oligomerization of the 42-residue amyloid β-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J. Biol. Chem. 272, 7977–7982 (1997).
Hsia, A. Y. et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse model. Proc. Natl Acad. Sci. USA 96, 3228–3233 (1999).
Mucke, L. et al. High-level neuronal expression of Aβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 (2000).
Hartley, D. M. et al. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 19, 8876–8884 (1999).
Xia, W. et al. Presenilin complexes with the C-terminal fragments of amyloid precursor protein at the sites of amyloid β-protein generation. Proc. Natl Acad. Sci. USA 97, 9299–9304 (2000).
Kim, J. H., Anwyl, R., Suh, Y. H., Djamgoz, M. B. & Rowan, M. J. Use-dependent effects of amyloidogenic fragments of β-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J. Neurosci. 21, 1327–1333 (2001).
Qiu, W. Q. et al. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J. Biol. Chem. 273, 32730–32738 (1998).
Lambert, M. P. et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl Acad. Sci. USA 95, 6448–6453 (1998).
Walsh, D. M. et al. Amyloid β-protein fibrillogenesis: structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 274, 25945–25952 (1999).
Chui, D.-H. et al. Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nature Med. 5, 560–564 (1999).
Chen, G. et al. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature 408, 975–979 (2000).
Lewis, J. et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293, 1487–1491 (2001).
Larson, J., Lynch, G., Games, D. & Seubert, P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices for young and aged PDAPP mice. Brain Res. 840, 23–35 (1999).
Moechars, D. et al. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 274, 6483–6492 (1999).
Chapman, P. F. et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neurosci. 2, 271–276 (1999).
Fitzhohn, S. M. et al. Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein. J. Neurosci. 21, 4691–4698 (2001).
Rochet, J. C. & Lansbury, P. T. Jr Amyloid fibrillogenesis: themes and variations. Curr. Opin. Struct. Biol. 10, 60–68 (2000).
Chiti, F. et al. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc. Natl Acad. Sci. USA 96, 3590–3590 (1999).
Chesneau, V. & Rosner, M. R. Functional human insulin-degrading enzyme can be expressed in bacteria. Protein Expr. Purif. 19, 91–98 (2000).
Getman, D. P. et al. Discovery of a novel class of potent HIV-1 protease inhibitors containing the (R)-(hydroxyethyl)urea isostere. J. Med. Chem. 36, 288–291 (1993).
Acknowledgements
We thank M. Rosner and V. Chesneau for the gift of the pProExH6HA IDE expression vector, B. Zheng for ELISA analysis, S. Mansourian for assistance in the preparation of illustrations and W. T. Kimberly, W. P. Esler and D. M. Hartley for discussions. Supported by NIH grants (to D.J.S. and M.S.W.) and by Enterprise Ireland and the Health Research Board Ireland (M.R. and R.A.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests
Rights and permissions
About this article
Cite this article
Walsh, D., Klyubin, I., Fadeeva, J. et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002). https://doi.org/10.1038/416535a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/416535a
This article is cited by
-
Alleviating the unwanted effects of oxidative stress on Aβ clearance: a review of related concepts and strategies for the development of computational modelling
Translational Neurodegeneration (2023)
-
Learnings about Aβ from human brain recommend the use of a live-neuron bioassay for the discovery of next generation Alzheimer’s disease immunotherapeutics
Acta Neuropathologica Communications (2023)
-
Unravelling the mechanotransduction pathways in Alzheimer’s disease
Journal of Biological Engineering (2023)
-
Iron response elements (IREs)-mRNA of Alzheimer's amyloid precursor protein binding to iron regulatory protein (IRP1): a combined molecular docking and spectroscopic approach
Scientific Reports (2023)
-
C3N nanodots inhibits Aβ peptides aggregation pathogenic path in Alzheimer’s disease
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.