Abstract
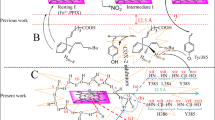
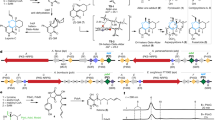
Cyclooxygenases are bifunctional enzymes that catalyse the first committed step in the synthesis of prostaglandins, thromboxanes and other eicosanoids1,2,3. The two known cyclooxygenases isoforms share a high degree of amino-acid sequence similarity1,2,3,4, structural topology5,6,7 and an identical catalytic mechanism1,2,3. Cyclooxygenase enzymes catalyse two sequential reactions in spatially distinct, but mechanistically coupled active sites8,9,10,11. The initial cyclooxygenase reaction converts arachidonic acid (which is achiral) to prostaglandin G2 (which has five chiral centres). The subsequent peroxidase reaction reduces prostaglandin G2 to prostaglandin H2. Here we report the co-crystal structures of murine apo-cyclooxygenase-2 in complex with arachidonic acid and prostaglandin. These structures suggest the molecular basis for the stereospecificity of prostaglandin G2 synthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Marnett,L. J., Rowlinson,S. W., Goodwin, D. C., Kalgutkar,A. S. & Lanzo,C. A. Arachidonic acid oxygenation by COX-1 and COX-2: Mechanisms of catalysis and inhibition. J. Biol. Chem. 274, 22903–22906 (1999).
Smith,W. L., Garavito,R. M. & DeWitt, D. L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 271, 33157– 33160 (1996).
Kulmacz,R. J. Cellular regulation of prostaglandin H synthase catalysis. FEBS Lett. 430, 154–157 ( 1998).
Herschman,H. R. Prostaglandin synthase 2. Biochim. Biophys. Acta 1299 , 125–140 (1996).
Picot,D., Loll,P. J. & Garavito, R. M. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature 367, 243–249 (1994).
Luong,C. et al. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nature Struct. Biol. 3, 927–933 (1996).
Kurumbail,R. G. et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti- inflammatory agents. Nature 384, 644–648 (1996); erratum ibid 385, 555 (1997).
Hamberg,M. & Samuelsson,B. On the mechanism of the biosynthesis of prostaglandins E-1 and F-1- alpha. J. Biol. Chem. 242, 5336–5343 (1967).
Dietz,R., Nastainczyk,W. & Ruf, H. H. Higher oxidation states of prostaglandin H synthase. Rapid electronic spectroscopy detected two spectral intermediates during the peroxidase reaction with prostaglandin G2. Eur. J. Biochem. 171, 321–328 (1988).
Smith,W. L., Eling,T. E., Kulmacz,R. J., Marnett,L. J. & Tsai,A. Tyrosyl radicals and their role in hydroperoxide-dependent activation and inactivation of prostaglandin endoperoxide synthase. Biochemistry 31, 3–7 ( 1992).
Kulmacz,R. J., Pendleton,R. B. & Lands, W. E. Interaction between peroxidase and cyclooxygenase activities in prostaglandin-endoperoxide synthase. Interpretation of reaction kinetics. J. Biol. Chem. 269, 5527– 5536 (1994).
Landino,L. M., Crews,B. C., Gierse,J. K., Hauser,S. D. & Marnett,L. J. Mutational analysis of the role of the distal histidine and glutamine residues of prostaglandin-endoperoxide synthase-2 in peroxidase catalysis, hydroperoxide reduction, and cyclooxygenase activation. J. Biol. Chem. 272, 21565– 21574 (1997).
Word,J. M. et al. Visualizing and quantitating molecular goodness-of-fit: small-probe contact dots with explicit hydrogens. J. Mol. Biol. 285, 1711–1733 (1998).
Karthein,R., Dietz,R., Nastainczyk,W. & Ruf,H. H. Higher oxidation states of prostaglandin H synthase. EPR study of a transient tyrosyl radical in the enzyme during the peroxidase reaction. Eur. J. Biochem. 171, 313–320 ( 1988).
Shimokawa,T., Kulmacz,R. J., DeWitt,D. L. & Smith,W. L. Tyrosine 385 of prostaglandin endoperoxide synthase is required for cyclooxygenase catalysis. J. Biol. Chem. 265, 20073– 20076 (1990).
Hamberg,M. Stereochemistry of oxygenation of linoleic acid catalyzed by prostaglandin-endoperoxide H synthase-2. Arch. Biochem. Biophys. 349, 376–380 (1998).
Eling,T. E., Glasgow,W. C., Curtis,J. F., Hubbard,W. C. & Handler,J. A. Studies on the reduction of endogenously generated prostaglandin G2 by prostaglandin H synthase. J. Biol. Chem. 266, 12348–12355 ( 1991).
Ueno,R., Shimizu,T., Kondo,K. & Hayaishi,O. Activation mechanism of prostaglandin endoperoxide synthetase by hemoproteins. J. Biol. Chem. 257, 5584–5588 ( 1982).
Bhattacharyya,D. K., Lecomte,M., Rieke,C. J., Garavito,M. & Smith,W. L. Involvement of arginine 120, glutamate 524, and tyrosine 355 in the binding of arachidonate and 2-phenylpropionic acid inhibitors to the cyclooxygenase active site of ovine prostaglandin endoperoxide H synthase-1. J. Biol. Chem. 271, 2179– 2184 (1996).
Rieke,C. J., Mulichak,A. M., Garavito, R. M. & Smith,W. L. The role of arginine 120 of human prostaglandin endoperoxide H synthase- 2 in the interaction with fatty acid substrates and inhibitors. J. Biol. Chem. 274, 17109–17114 (1999).
Rowlinson,S. W., Crews,B. C., Lanzo,C. A. & Marnett,L. J. The binding of arachidonic acid in the cyclooxygenase active site of mouse prostaglandin endoperoxide synthase-2 (COX-2): a putative L-shaped binding conformation utilizing the top channel region. J. Biol. Chem. 274 , 23305–23310 (1999).
Hsi,L. C. et al. Trp387 and the putative leucine zippers of PGH synthases-1 and -2. J. Lipid. Mediat. 6, 131– 138 (1993).
Tsai,A., Palmer,G., Xiao,G., Swinney,D. C. & Kulmacz, R. J. Structural characterization of arachidonyl radicals formed by prostaglandin H synthase-2 and prostaglandin H synthase-1 reconstituted with mangano protoporphyrin IX. J. Biol. Chem. 273, 3888–3894 (1998).
Stevens,A. M. et al. Crystallization of recombinant cyclo-oxygenase-2. J. Cryst. Growth 196, 350–355 (1999).
Otwinowski,Z. & Minor,W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276 , 307–326 (1997).
Brünger,A. T. X-PLOR version 3. 1: A system for X-ray crystallography and NMR. Yale University Press, New Haven, CT, USA (1992).
Jiang,J. -S. & Brünger,A. T. Protein hydration observed by X-ray diffraction. Solvation properties of penicillopepsin and neuraminidase crystal structures. J. Mol. Biol. 243, 100 –115 (1994).
Read,R. J. Improved Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A 42, 140 –149 (1986).
Jones,T. A., Zou,J. -Y. & Cowan,S. W. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 ( 1991).
Capdevila,J. H. et al. The catalytic outcomes of the constitutive and the mitogen inducible isoforms of prostaglandin H2 synthase are markedly affected by glutathione and glutathione peroxidase(s). Biochemistry 34, 3325–3337 (1995).
Acknowledgements
We are grateful to M. C. Walker, R. Fletterick, T. Rydel, H.-S. Shieh, J. Pierce, P. C. Isakson and K. Seibert for substantive discussions. LC–MS experiments were performed by J. Muhammad. Figures were generated with RIBBONS (M. Carson). S.W.R. is the recipient of an Australian National Health and Medical Research Council C.J. Martin Fellowship.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 879 kb)
Rights and permissions
About this article
Cite this article
Kiefer, J., Pawlitz, J., Moreland, K. et al. Structural insights into the stereochemistry of the cyclooxygenase reaction . Nature 405, 97–101 (2000). https://doi.org/10.1038/35011103
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35011103
This article is cited by
-
Explainable artificial intelligence-assisted virtual screening and bioinformatics approaches for effective bioactivity prediction of phenolic cyclooxygenase-2 (COX-2) inhibitors using PubChem molecular fingerprints
Molecular Diversity (2024)
-
Dual synergistic inhibition of COX and LOX by potential chemicals from Indian daily spices investigated through detailed computational studies
Scientific Reports (2023)
-
Dietary intake of α-ketoglutarate ameliorates α-synuclein pathology in mouse models of Parkinson’s disease
Cellular and Molecular Life Sciences (2023)
-
In silico molecular docking of cyclooxygenase (COX-2), ADME-toxicity and in vitro evaluation of antioxidant and anti-inflammatory activities of marine macro algae
3 Biotech (2023)
-
Design and synthesis of new 1,4,5-trisubstituted triazole-bearing benzenesulphonamide moiety as selective COX-2 inhibitors
Medicinal Chemistry Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.