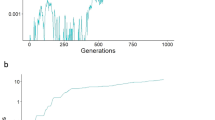
Abstract
Why sex prevails in nature remains one of the great puzzles of evolution1,2. Sexual reproduction has an immediate cost relative to asexual reproduction, as males only express their contribution to population growth through females. With no males to sustain, an asexual mutant can double its relative representation in the population in successive generations. This is the widely accepted ‘twofold cost of males’1,2,3. Many studies4,5,6,7 have attempted to explain how sex can recoup this cost from fitness benefits associated with the recombination of parental genotypes, but these require complex biological environments that cycle over evolutionary timescales. In contrast, we have considered the ecological dynamics that govern asexual invasion. Here we show the existence of a threshold growth rate for the sexual population, above which the invasion is halted by intraspecific competition. The asexual population then exerts a weaker inhibitory effect on the carrying capacity of the sexual population than on its own carrying capacity. The stable outcome of this is coexistence on a depleted resource base. Under these ecological circumstances, longer-term benefits of sex may eventually drive out the asexual competitor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Williams, G. C. Sex and Evolution (Princeton Univ. Press, Princeton, 1975).
Ridley, M. Evolution 2nd edn (Blackwell Science, Oxford, 1996).
Maynard Smith, J. The Evolution of Sex (Cambridge Univ. Press, Cambridge, UK, 1978).
Kondrashov, A. S. Classification of hypotheses on the advantage of amphimixis. J. Hered. 84, 372–387 ( 1993).
Judson, O. P. & Normark, B. B. Ancient asexual scandals. Trends Ecol. Evol. 11, 41–46 (1996).
Hurst, L. D. & Peck, J. R. Recent advances in understanding of the evolution and maintenance of sex. Trends Ecol. Evol. 11, 46–52 (1996).
Hamilton, W. D., Axelrod, R. & Tanese, R. Sexual reproduction as an adaptation to resist parasites. Proc. Natl Acad. Sci. USA 87, 3566– 3573 (1990).
Volterra, V. in Animal Ecology (ed. Chapman, R. N.) 409–448 (McGraw Hill, New York, 1931).
Lotka, A. J. The growth of mixed populations: two species competing for a common food supply. J. Wash. Acad. Sci. 22, 461– 469 (1932).
Begon, M., Harper, J. L. & Townsend, C. R. Ecology 3rd edn (Blackwell Science, Oxford, 1996).
Vrijenhoek, R. C. Factors affecting clonal diversity and coexistence. Am. Zool. 19, 787–797 (1979).
Bell, G. The Masterpiece of Nature (Croom Helm, London, 1982 ).
Browne, R. A. & Hoopes, C. W. Genotype diversity and selection in asexual brine shrimp (Artemia). Evolution 44, 1035–1051 (1990).
Bolger, D. T. & Case, T. J. Divergent ecology of sympatric clones of the asexual gecko, Lepidodactylus lugubris. Oecologia 100, 397–405 ( 1994).
Semlitsch, R. D., Hotz, H. & Guex, G. D. Competition among tadpoles of coexisting hemiclones of hybridogenetic Rana esculenta: support for the frozen niche variation model. Evolution 51, 1249– 1261 (1997).
Weeks, A. R. & Hoffmann, A. A. Intense selection of mite clones in a heterogeneous environment. Evolution 52, 1325–1333 (1998).
Jokela, J., Lively, C. M., Fox, J. A. & Dybdahl, M. F. Flat reaction norms and “frozen” phenotypic variation in clonal snails (Potamopyrgus antipodarum). Evolution 51, 1120–1129 (1997).
Vjijenhoek, R. C. & Pfeiler, E. Differential survival of sexual and asexual Poeciliopsis during environmental stress. Evolution 51, 1593–1600 (1997).
Case, T. J. Patterns of coexistence in sexual and asexual species of Cnemidophorus lizards. Oecologia 83, 220– 227 (1990).
Doebeli, M. An explicit genetic model for ecological character displacement. Ecology 77, 510–520 ( 1996).
Skelton, P. (ed.) Evolution: a Biological and Palaeontological Approach (Addison-Wesley, Wokingham, UK, 1993).
Bulmer, M. Theoretical Evolutionary Ecology (Sinauer, Sunderland, Massachusetts, 1994).
Futuyma, D. J. Evolutionary Biology 3rd edn (Sinauer, Sunderland, Massachusetts, 1998).
Maynard Smith, J. Evolutionary Genetics 2nd edn (Oxford Univ. Press, Oxford, 1998).
Stearns, S. C. & Hoekstra, R. F. Evolution: an Introduction (Oxford Univ. Press, Oxford, 2000).
Case, T. J. & Taper, M. L. On the coexistence and coevolution of asexual and sexual competitors. Evolution 40, 366–387 (1986).
Levins, R. in Lectures on Mathematics in the Life Sciences, vol. 2 (ed. Gerstenhaber, M.) 75–107 (American Mathematical Society, Providence, 1970).
Connolly, S. R. & Roughgarden, J. Theory of marine communities: competition, predation, and recruitment-dependent interaction strength. Ecol. Monogr. 69, 277– 296 (1999).
Lively, C. M. Host-parasite coevolution and sex. BioScience 46, 107–114 (1996).
Acknowledgements
We thank G. F. Turner, S. J. Hawkins and C. P. Please for clarifications of key issues. This work was supported by a Natural Environment Research Council grant to C.P.D., and by an Engineering and Physical Sciences Research Council studentship to G.E.P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doncaster, C., Pound, G. & Cox, S. The ecological cost of sex. Nature 404, 281–285 (2000). https://doi.org/10.1038/35005078
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35005078
This article is cited by
-
Apomixis and genetic background affect distinct traits in Hieracium pilosella L. grown under competition
BMC Biology (2021)
-
Sperm-dependent asexual hybrids determine competition among sexual species
Scientific Reports (2019)
-
Life cycle and population genetics of bird cherry-oat aphids Rhopalosiphum padi in China: an important pest on wheat crops
Journal of Pest Science (2017)
-
Differences in resource assimilation between the unisexual Amazon molly, Poecilia formosa (Poeciliidae) and its sexual host (Poecilia latipinna)
Environmental Biology of Fishes (2014)
-
Do ecological niches differ between sexual and asexual lineages of an aphid species?
Evolutionary Ecology (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.