Abstract
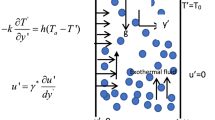
There is a remarkable difference between the maximum temperature of black smoker effluent (350 °C–400 °C) and the temperature of the solidifying magma which heats it (∼1,200 °C)1,2,3. It has been suspected4 for some time that the nonlinear thermodynamic properties of water5 might be responsible for this discrepancy. Here, we translate this hypothesis into a physical model, by examining the internal temperature structure of convection cells in a porous medium. We demonstrate that, at pressures appropriate to seafloor crust, plumes of pure water form naturally at ∼400 °C for any heat source with temperature greater than ∼500 °C. Higher temperatures are confined to a boundary layer at the base of the convection cell, where the flow is horizontal. The phenomenon is explained analytically using the thermodynamic properties of water, and is illustrated by numerical simulations. Our model predicts the existence of the high-temperature ‘reaction zone’ found in ophiolites6 and suggests that vent temperatures will remain steady as magma chambers solidify and cool7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lister, C. R. B. Heat transfer between magmas and hydrothermal systems, or, six lemmas in search of a theorem. Geophys. J. Int. 120, 45–59 (1995).
Lowell, R. P., Rona, P. A. & Von Herzen, R. P. Seafloor hydrothermal systems. J. Geophys. Res. 100, 327–352 ( 1995).
Wilcock, W. S. D. Cellular convection models of mid-ocean ridge hydrothermal circulation and the temperatures of black smoker fluids. J. Geophys. Res. 103, 2585–2596 (1998).
Bischoff, J. L. & Rosenbauer, R. J. An empirical equation of state for hydrothermal seawater (3. 2 percent NaCl). Amer. J. Sci. 285, 725–763 (1985).
Johnson, J. W. & Norton, D. Critical phenomena in hydrothermal systems: state, thermodynamic, electrostatic, and transport properties of H2O in the critical region. Am. J. Sci. 291, 541–648 (1991).
Gillis, K. M. & Roberts, M. D. Cracking at the magma-hydrothermal transition: evidence from the Troodos Ophiolite, Cyprus. Earth Planet. Sci. Lett. 169, 227–244 (1999).
Lowell, R. P. & Germanovich, L. N. On the temporal evolution of high-temperature hydrothermal systems at ocean ridge crests. J. Geophys. Res. 99, 565–575 (1994).
Lister, C. R. B. On the penetration of water into hot rock. Geophys. J. R. Astron. Soc. 39, 465–509 ( 1974).
Fournier, R. O. The transition from hydrostatic to greater than hydrostatic fluid pressure in presently active continental hydrothermal systems in crystalline rock. Geophys. Res. Lett. 18, 955– 958 (1991).
Lowell, R. P., Van Cappellen, P. & Germanovich, L. Silica precipitation in fractures and the evolution of permeability in hydrothermal upflow zones. Science 260, 192–194 (1993).
Germanovich, L. N. & Lowell, R. P. Percolation theory, thermoelasticity, and discrete hydrothermal venting in the Earth's crust. Science 255, 1564– 1567 (1992).
Straus, J. M. & Schubert, G. Thermal convection of water in a porous medium: effects of temperature- and pressure-dependent thermodynamic and transport properties. J. Geophys. Res. 82, 325–333 (1977).
Dunn, J. C. & Hardee, H. C. Superconvecting geothermal zones. J. Volcanol. Geotherm. Res. 11, 189– 201 (1981).
Ingebritsen, S. E. & Hayba, D. O. Fluid flow and heat transport near the critical point of H2O. Geophys. Res. Lett. 21, 2199–2202 (1994).
Norton, D. & Knight, J. Transport phenomena in hydrothermal systems: cooling plutons. Am. J. Sci. 277, 937–981 (1977).
Hayba, D. O. & Ingebritsen, S. E. The Computer Model HYDROTHERM, a Three-dimensional Finite-difference Model to Simulate Ground-water Flow and Heat Transport in the Temperature Range of 0 to 1,200 degrees Celsius. (US Geological Survey Water-Resources Investigations Report, 94-4045, Denver, Colorado, 1994).
Dickson, P., Schultz, A. & Woods, A. in Hydrothermal Vents and Processes (ed. Parson, L. M. et al.) Vol. 87, 145–157 (Geological Society Special Publication, London, 1995).
Faust, C. R. & Mercer, J. W. Geothermal reservoir simulation 1. Mathematical models for liquid- and vapor-dominated hydrothermal systems. Wat. Resour. Res. 15, 23– 30 (1979).
Haar, L., Gallagher, J. S. & Kell, G. S. NBS/NRC Steam Tables, 1– 320 (Hemisphere, New York, 1984).
Watson, J. T. R., Basu, R. S. & Sengers, J. V. An improved representative equation for the dynamic viscosity of water substance. J. Phys. Chem. Ref. Data 9, 1255–1290 (1980).
Sengers, J. V. & Kamgar-Parsi, B. Representative equations for the viscosity of water substance. J. Phys. Chem. Ref. Data 13, 185–205 ( 1984).
Sengers, J. V. & Watson, J. T. R. Improved international formulation for the viscosity and thermal conductivity of water substance. J. Phys. Chem. Ref. Data 15, 1291–1314 (1986).
Elder, J. E. Geothermal Systems 180–187 (Academic, London, 1981).
Pascoe, A. R. & Cann, J. R. in Hydrothermal Vents and Processes (ed. Parson, L. M. et al.) Vol. 87, 159– 173 (Geological Society Special Publication, London, 1995).
Anderko, A. & Pitzer, K. S. Equation-of-state representation of phase equilibria and volumetric properties of the system NaCl-H2O above 573 K. Geochim. Cosmochim. Acta. 57, 1657–1680 (1993).
Lapwood, E. R. Convection of a fluid in a porous medium. Proc. Camb. Phil. Soc. 44, 508–521 ( 1948).
Phillips, O. M. Flow and Reactions in Permeable Rocks 245–264 (Cambridge Univ. Press, 1991).
Acknowledgements
We thank USGS for permission to use HYDROTHERM. The calculations were carried out on the Enigma high-performance computing centre at the Institute of Theoretical Geophysics. We acknowledge the support of that facility by the Higher Education Research Foundation for England. T.J. is supported by a studentship from the Natural Environment Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jupp, T., Schultz, A. A thermodynamic explanation for black smoker temperatures. Nature 403, 880–883 (2000). https://doi.org/10.1038/35002552
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35002552
This article is cited by
-
Brine Formation and Mobilization in Submarine Hydrothermal Systems: Insights from a Novel Multiphase Hydrothermal Flow Model in the System H2O–NaCl
Transport in Porous Media (2021)
-
Implementation Strategies for Accurate and Efficient Control Volume-Based Two-Phase Hydrothermal Flow Solutions
Transport in Porous Media (2018)
-
Geologic controls on supercritical geothermal resources above magmatic intrusions
Nature Communications (2015)
-
Numerical simulations of mid-ocean ridge hydrothermal circulation including the phase separation of seawater
Earth, Planets and Space (2014)
-
Hybrid shallow on-axis and deep off-axis hydrothermal circulation at fast-spreading ridges
Nature (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.