Abstract
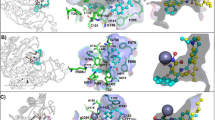
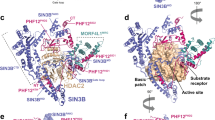
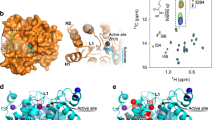
Histone deacetylases (HDACs) mediate changes in nucleosome conformation and are important in the regulation of gene expression1. HDACs are involved in cell-cycle progression and differentiation, and their deregulation is associated with several cancers2,3. HDAC inhibitors, such as trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA), have anti-tumour effects, as they can inhibit cell growth4,5,6, induce terminal differentiation4,5 and prevent the formation of tumours in mice models7,8, and they are effective in the treatment of promyelocytic leukemia3. Here we describe the structure of the histone deacetylase catalytic core, as revealed by the crystal structure of a homologue from the hyperthermophilic bacterium Aquifex aeolicus, that shares 35.2% identity with human HDAC1 over 375 residues, deacetylates histones in vitro and is inhibited by TSA and SAHA. The deacetylase, deacetylase–TSA and deacetylase–SAHA structures reveal an active site consisting of a tubular pocket, a zinc-binding site and two Asp–His charge-relay systems, and establish the mechanism of HDAC inhibition. The residues that make up the active site and contact the inhibitors are conserved across the HDAC family. These structures also suggest a mechanism for the deacetylation reaction and provide a framework for the further development of HDAC inhibitors as anti-tumour agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Davie,J. R. & Chadee,D. N. Regulation and regulatory parameters of histone modifications. J. Cell Biochem. (Suppl.) 30–31, 203–213 (1998).
Kouzarides,T. Histone acetylases and deacetylases in cell proliferation. Curr. Opin. Genet. Dev. 9, 40–84 (1999).
Fenrick,R. & Hiebert,S. W. Role of histone deacetylases in acute leukemia. J. Cell. Biochem. (Suppl.) 30–31, 194–202 (1998).
Yoshida,M., Horinouchi,S. & Beppu,T. Trichostatin A and trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function. Bioessays 17, 423–430 (1995).
Richon,V. M. et al. Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc. Natl Acad. Sci. USA 93, 5705–5708 (1996).
Richon,V. M. et al. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc. Natl Acad. Sci. USA 95, 3003–3007 (1998).
Cohen,L. A. et al. Inhibition of N-methylnitrosourea-induced tumours by the cytodifferentiating agent, suberanilohydroxamic acid (SAHA). Proc. AACR 39, 108, abstr. 736 (1998).
Desai,D., El-Bayoumy,K. & Amin,S. Chemopreventive efficacy of suberanilohydroxamic acid (SAHA), a cytodifferentiating agent, against tobacco-specific nitrosamine 4-(methylinitros-amino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in female A/J mice. Proc. AACR 40, 2396, abstr. 362 (1999).
Struhl,K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12, 599–606 (1998).
Taunton,J. Hassig,C. A. & Schreiber,S. L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272, 408–411 (1996).
Grozinger,C. M., Hassig,C. A. & Schreiber,S. L. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl Acad. Sci. USA 96, 4868–4873 (1999).
Fischle,W. et al. A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1p. J. Biol. Chem. 274, 11713–11720 (1999).
Leipe,D. D. & Landsman,D. Histone deacetylases, acetoin utilization proteins and acetylpolyamine amidohydrolases are members of an ancient protein superfamily. Nucleic Acids Res. 25, 3693–3697 (1997).
Hassig,C. A. et al. A role for histone deacetylase activity in HDAC1-mediated transcriptional repression. Proc. Natl Acad. Sci. USA 95, 3519–3524 (1998).
Fersht,A. R. & Sperling,J. The charge relay system in chymotrypsin and chymotrypsinogen. J. Mol. Biol. 74, 137–149 (1973).
Chothia,C. & Lesk,A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 5, 823–826 (1986).
Grams,F. et al. Structure determination and analysis of human neutrophil collagenase complexed with a hydroxamate inhibitor. Biochemistry 34, 14012–14020 (1995).
Lovejoy,B. et al. Crystal structures of MMP-1 and -13 reveal the structural basis for selectivity of collagenase inhibitors. Nature Struct. Biol. 6, 217–221 (1999).
Holmes,M. A. & Matthews,B. W. Binding of hydroxamic acid inhibitors to crystalline thermolysin suggests a pentacoordinate zinc intermediate in catalysis. Biochemistry 20, 6912–6920 (1981).
Shute,R. E., Dunlap,B. & Rich,D. H. Analogues of the cytostatic and antimitogenic agents chlamydocin and HC-toxin: synthesis and biological activity of chloromethyl ketone and diazomethyl ketone functionalized cyclic tetrapeptides. J. Med. Chem. 30, 71–78 (1987).
Christianson,D. W. & Lipscomb,W. N. Carboxypeptidase A. Acc. Chem. Res. 22, 62–69 (1989).
Kadosh,D. & Struhl,K. Histone deacetylase activity of Rpd3 is important for transcriptional repression in vivo. Genes Dev. 12, 797–805 (1998).
The CCP4 suite: Programs for computational crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Jones,T. A., Zou,J. Y., Cowan,S. W. & Kjeldgaard,M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brunger,A. T. et al. Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallgr. D 54, 905–921 (1998).
Hendzel,M. J., Delcuve,G. P. & Davie,J. R. Histone deacetylase is a component of the internal nuclear matrix. J. Biol. Chem. 266, 21936–21942 (1991).
Deckert,G. et al. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature 392, 353–358 (1998).
Kraulis,P. Molscript: A program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Merritt,E. A. & Bacon,D. J. Raster3D-Photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997).
Kanyo,Z. F., Scholnick,L. R., Ash,D. E. & Christianson,D. W. Structure of a unique binuclear manganes cluster in arginase. Nature 383, 554–557 (1996).
Acknowledgements
We thank R. Huber for the A. aeolicus chromosomal DNA preparation; C. A. Hassig and S. L. Schreiber for the HDAC1-FLAG baculovirus and for helpful discussions; A. S. Mildvan and J. Thornson for advice on the reaction mechanism; and C. Murray for administrative help. Supported by the Howard Hughes Medical Institute, the NIH, the Dewitt Wallace Foundation and the Samuel and May Rudin Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Finnin, M., Donigian, J., Cohen, A. et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401, 188–193 (1999). https://doi.org/10.1038/43710
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/43710
This article is cited by
-
Emerging therapeutic strategies in cancer therapy by HDAC inhibition as the chemotherapeutic potent and epigenetic regulator
Medical Oncology (2024)
-
Progress in discovery and development of natural inhibitors of histone deacetylases (HDACs) as anti-cancer agents
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Pharmacophore-based virtual screening of ZINC database, molecular modeling and designing new derivatives as potential HDAC6 inhibitors
Molecular Diversity (2023)
-
Characteristics and mechanisms of latency-reversing agents in the activation of the human immunodeficiency virus 1 reservoir
Archives of Virology (2023)
-
Recent advancement of HDAC inhibitors against breast cancer
Medical Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.