Abstract
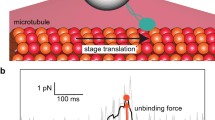
Atomic force microscopy (AFM)1, 2 has been used to measure the strength of bonds between biological receptor molecules and their ligands3,4,5,6. But for weak noncovalent bonds, a dynamic spectrum of bond strengths is predicted as the loading rate is altered, with the measured strength being governed by the prominent barriers traversed in the energy landscape along the force-driven bond-dissociation pathway7. In other words, the pioneering early AFM measurements represent only a single point in a continuous spectrum of bond strengths, because theory predicts that these will depend on the rate at which the load is applied. Here we report the strength spectra for the bonds between streptavidin (oravidin) and biotin8—the prototype of receptor–ligand interactions used in earlier AFM studies3,4,5, and which have been modelled by molecular dynamics9, 10. We have probed bond formation over six orders of magnitude in loading rate, and find that the bond survival time diminished from about 1 min to 0.001 s with increasing loading rate over this range. The bond strength, meanwhile, increased from about 5 pN to 170 pN. Thus, although they are among the strongest noncovalent linkages in biology (affinity of 1013 to 1015 M−1)8, 11, these bonds in fact appear strong or weak depending on how fast they are loaded. We are also able to relate the activation barriers derived from our strength spectra to the shape of the energy landscape derived from simulations of the biotin–avidin complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Binnig, G., Quate, C. F. & Gerber, C. H. Atomic force microscopy. Phys. Rev. Lett. 56, 930–933 ( 1986).
Drake, B. et al. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science 243, 1586– 1589 (1989).
Lee, G. U., Kidwell, D. A. & Colton, R. J. Sensing discrete streptavidin-biotin interactions with atomic force microscopy. Langmuir 10, 354–357 (1994).
Florin, E.-L., Moy, V. T. & Gaub, H. E. Adhesive forces between individual ligand receptor pairs. Science 264, 415–417 (1994).
Moy, V. T., Florin, E.-L. & Gaub, H. E. Intermolecular forces and energies between ligands and receptors. Science 264, 257– 259 (1994).
Hinterdorfer, P., Baumgartner, W., Gruber, H. J., Schilcher, K. & Schindler, H. Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc. Natl Acad. Sci. USA 93, 3477– 3481 (1996).
Evans, E. & Ritchie, K. Dynamic strength of molecular adhesion bonds. Biophys. J. 72, 1541– 1555 (1997).
Green, N. M. Avidin. Adv. Protein Chem. 29, 85– 133 (1975).
Grubmuller, H., Heymann, B. & Tavan, P. Ligand binding: molecular mechanics calculation of the streptavidin-biotin rupture force. Science 271, 997–999 (1996).
Izrailev, S., Stepaniants, S., Balsera, M., Oono, Y. & Schulten, K. Molecular dynamics study of unbinding of the avidin-biotin complex. Biophys. J. 72, 1568–1581 (1997).
Chilkoti, A. & Stayton, P. S. Molecular origins of the slow streptavidin-biotin dissociation kinetics. J.Am. Chem. Soc. 117, 10622–10628 (1995).
Evans, E., Ritchie, K. & Merkel, R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys. J. 68, 2580–2587 (1995).
Bell, G. I. Models for the specific adhesion of cells to cells. Science 200, 618–627 (1978).
Weber, P. C., Ohlendorf, D. H., Wendoloski, J. J. & Salemme, F. R. Structural origins of high-affinity biotin binding to streptavidin. Science 243, 85–88 ( 1989).
Livnah, O., Bayer, E. A., Wilchek, M. & Sussman, J. L. Three-dimensional structures of avidin and the avidin-biotin complex. Proc. Natl Acad. Sci. USA 90, 5076– 5080 (1993).
Freitag, S., Le Trong, I., Klumb, L., Stayton, P. S. & Stenkamp, R. E. Structural studies of the streptavidin binding loop. Protein Sci. 6, 1157–1166 (1997).
Chu, V., Freitag, S., Le Trong, I., Stenkamp, R. E. & Stayton, P. S. Thermodynamic and structural consequences of flexible loop deletion by circular permutation in the streptavidin-biotin system. Protein Sci. 7, 848– 859 (1998).
Alon, R., Hammer, D. A. & Springer, T. A. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature 374, 539–542 (1995).
Brunk, D. K., Goetz, D. J. & Hammer, D. A. Sialyl Lewisx /E-selectin-mediated rolling in a cell-free system. Biophys. J. 71, 2902–2907 (1996).
Rief, M., Gautel, M., Osterhelt, F., Fermandez, J. M. & Gaub, H. E. Reversible unfolding of individual titin immunoglobin domains by AFM. Science 276, 1109–1112 (1997).
Oberhauser, A. F., Marszalek, P. E., Erickson, H. P. & Fernandez, J. M. The molecular elasticity of the extracellular matrix protein tenascin. Nature 393, 181–185 ( 1998).
Kramers, H. A. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica (Utrecht) 7, 284– 304 (1940).
Hanggi, P., Talkner, P. & Borkovec, M. Reaction-rate theory: fifty years after Kramers. Rev. Mod. Phys. 62, 251–342 (1990).
Wong, S. S., Joselivich, E., Woolley, A. T., Cheung, C. L. & Lieber, C. M. Covalently functionalized nanotubes as nanometre-sized probes in chemistry and biology. Nature 394, 52–55 (1998).
Acknowledgements
We thank A. Chilkoti, C. Cantor and the group of K. Schulten for helpful discussions. The work was supported by USPHS National Institutes of Health, Medical Research Council of Canada, and the Canadian Institute for Advanced Research Program in Science of Soft Surfaces and Interfaces.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merkel, R., Nassoy, P., Leung, A. et al. Energy landscapes of receptor–ligand bonds explored with dynamic force spectroscopy. Nature 397, 50–53 (1999). https://doi.org/10.1038/16219
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/16219
This article is cited by
-
Phosphorylation disrupts long-distance electron transport in cytochrome c
Nature Communications (2022)
-
Calculating the force-dependent unbinding rate of biological macromolecular bonds from force-ramp optical trapping assays
Scientific Reports (2022)
-
Low cost and massively parallel force spectroscopy with fluid loading on a chip
Nature Communications (2022)
-
Size compatibility and concentration dependent supramolecular host–guest interactions at interfaces
Nature Communications (2022)
-
Microfabricated cantilevers for parallelized cell-cell adhesion measurements
European Biophysics Journal (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.