Abstract
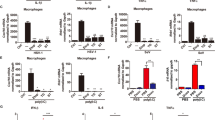
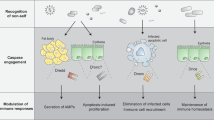
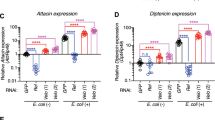
Induction of the adaptive immune response depends on the expression of co-stimulatory molecules and cytokines by antigen-presenting cells. The mechanisms that control the initial induction of these signals upon infection are poorly understood. It has been proposed that their expression is controlled by the non-clonal, or innate, component of immunity that preceded in evolution the development of an adaptive immune system in vertebrates1. We report here the cloning and characterization of a human homologue of the Drosophila toll protein (Toll) which has been shown to induce the innate immune response in adult Drosophila2,3,4. Like Drosophila Toll, human Toll is a type I transmembrane protein with an extracellular domain consisting of a leucine-rich repeat (LRR) domain, and a cytoplasmic domain homologous to the cytoplasmic domain of the human interleukin (IL)-1 receptor. Both Drosophila Toll and the IL-1 receptor are known to signal through the NF-κB pathway5,6,7. We show that a constitutively active mutant of human Toll transfected into human cell lines can induce the activation of NF-κB and the expression of NF-κB-controlled genes for the inflammatory cytokines IL-1, IL-6 and IL-8, as well as the expression of the co-stimulatory molecule B7.1, which is required for the activation of naive T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Janeway, C. A. J Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp. Quant. Biol. 54, 1–13 (1989).
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. The dorsoventral regulatory gene cassette spatzle/toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996).
Rosetto, M., Engström, Y., Baldari, C. T., Telford, J. L. & Hultmark, D. Signals from the IL-1 receptor homolog, Toll, can activate an immune response in a Drosophila hemocyte cell line. Biochem. Biophys. Res. Commun. 209, 111–116 (1995).
Ip, Y. T. & Levine, M. Molecular genetics of Drosophila immunity. Curr. Opin. Genet. Dev. 4, 672–677 (1994).
Wasserman, S. A. Aconserved signal transduction pathway regulating the activity of rel-like proteins dorsal and NF-κB. Mol. Biol. Cell 4, 767–771 (1993).
Hultmark, D. Insect immunology: Ancient relationships. Nature 367, 116–117 (1994).
Belvin, M. P. & Anderson, K. V. Aconserved signaling pathway: the Drosophila Toll–dorsal pathway. Annu. Rev. Cell Dev. Biol. 12, 393–416 (1996).
Morisato, D. & Anderson, K. V. The spatzle gene encodes a component of the extracellular signaling pathway establishing the dorsal–ventral pattern of the Drosophila embryo. Cell 76, 677–688 (1994).
Gay, N. J. & Keith, F. J. Drosophila Toll and IL-1 receptor. Nature 351, 355–356 (1991).
Whitham, S. et al. The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell 78, 1101–1115 (1994).
Altschul, S. F., Boguski, M. S., Gish, W. & Wootton, J. C. Issues in searching molecular sequence databases. Nature Genet. 6, 119–129 (1994).
Lennon, G., Auffray, C., Polymeropoulos, M. & Soares, M. B. The IMAGE Consortium: an integrated molecular analysis of genomes and their expression. Genomics 33, 151–152 (1996).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: A Laboratory Manual(Cold Spring Harbor Laboratory Press, New York, (1989)).
Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J. (eds) PCR Protocols: A Guide to Methods and Applications(Academic, Orlando, (1992)).
Miyake, K., Yamashita, Y., Ogata, M., Sudo, T. & Kimoto, M. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J. Immunol. 154, 3333–3340 (1995).
Schneider, D. S., Hudson, K. L., Lin, T. Y. & Anderson, K. V. Dominant and recessive mutations define functional domains of Toll, a transmembrane protein required for dorsal–ventral polarity in the Drosophila embryo. Genes Dev. 5, 797–807 (1991).
Takahashi, N., Takahashi, Y. & Putnam, F. W. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc. Natl Acad. Sci. USA 82, 1906–1910 (1985).
Winans, K. A. & Hashimoto, C. Ventralization of the Drosophila embryo by deletion of extracellular leucine-rich repeats in the Toll protein. Mol. Biol. Cell. 6, 587–596 (1995).
Fearon, D. T. & Locksley, R. M. The instructive role of innate immunity in the acquired immune response. Science 272, 50–53 (1996).
Medzhitov, R. & Janeway, C. A. J On the semantics of immune recognition. Res. Immunol. 147, 208–214 (1996).
Yamamura, M. et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254, 277–279 (1991).
Acknowledgements
We thank our colleagues for discussions; P. G. Waterbury for DNA sequencing; S.Ghosh and R. Voll for pB2XLuc plasmid and for helpful suggestions; and J. Flaxenberg for assistance. We acknowledge the Howard Hughes Medical Institute and the NIAID, NIH for support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997). https://doi.org/10.1038/41131
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/41131
This article is cited by
-
Study on the mechanism of action of Wu Mei Pill in inhibiting rheumatoid arthritis through TLR4-NF-κB pathway
Journal of Orthopaedic Surgery and Research (2024)
-
Trichinella spiralis galectin binding to toll-like receptor 4 induces intestinal inflammation and mediates larval invasion of gut mucosa
Veterinary Research (2023)
-
Exploring the immune interactions between Oncomelania hupensis and Schistosoma japonicum, with a cross-comparison of immunological research progress in other intermediate host snails
Parasites & Vectors (2023)
-
Effect of glutamine on the systemic innate immune response in broiler chickens challenged with Salmonella pullorum
BMC Veterinary Research (2023)
-
PSMC5 regulates microglial polarization and activation in LPS-induced cognitive deficits and motor impairments by interacting with TLR4
Journal of Neuroinflammation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.