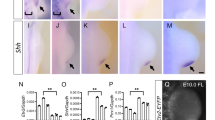
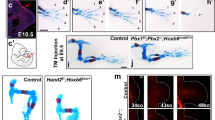
Abstract
Vertebrate limb outgrowth requires a structure called the apical ectodermal ridge, formation of which follows the previous establishment of the dorsoventral limb axis. Radical fringe is expressed in the dorsal ectoderm before the ridge appears, and is repressed by Engrailed-I, which is expressed in the ventral ectoderm. Misexpression of these genes indicates that a ridge is formed wherever there is a boundary between cells expressing and not expressing Radical fringe. Thus, as in Drosophila, Radical fringe positions the ridge at the dorsoventral limb boundary.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Saunders, J. W. & Gasseling, M. T. in Epithelial-Mesenchymal Interactions (eds Fleischmajer, R. & Billingham, R. E.) 78–97 (Baltimore, 1968).
Tickle, C., Summerbell, D. & Wolpert, L. Positional signalling and specification of digits in chick limb morphogenesis. Nature 254, 199–202 (1975).
Pautou, M. P. & Kieny, M. Interaction ec-mesodermique dans l'establissement de la polarité dorso-ventrale du pied de l'embryon de poulet. C. r. hebd Séanc. Acad. Sci., Paris D 277, 1225–1228 (1973).
MacCabe, J. A., Errick, J. E. & Saunders, J. W. J. Ectodermal control of the dorso-ventral axis in the leg bud of the chick embryo. Dev. Biol. 39, 69–82 (1974).
Saunders, J. W. J. The proximo-distal sequence of the origin of the parts of the chick wing and the role of the ectoderm. J. Exp. Zool. 108, 363–404 (1948).
Todt, W. & Fallon, J. F. Development of the apical ectodermal ridge in the chick wing bud. J. Embryol. Exp. Morphol. 80, 21–41 (1984).
Duboule, D. How to make a limb? Science 266, 575–576 (1994).
Maden, M. The limb bud—part two. Nature 371, 560–561 (1994).
Martin, G. Why thumbs are up. Nature 374, 410–411 (1995).
Tabin, C. The initiation of the limb bud: Growth factors, Hox genes, and retinoids. Cell 80, 671–674 (1995).
Parr, B. A. & McMahon, A. P. Dorsalizing signal Wnt-7a required for normal polarity of D–V and A–P axes of mouse limb. Nature 374, 350–353 (1995).
Yang, Y. Z. & Niswander, L. Interaction between the signaling molecules Wnt7a and shh during vertebrate limb development-dorsal signals regulated anteroposterior patterning. Cell 80, 939–947 (1995).
Riddle, R. et al. Induction of the LIM Homeobox gene Lmx-1 by Wnt7a establishes dorsoventral pattern in the vertebrate limb. Cell 83, 631–640 (1995).
Vogel, A., Rodriguez, C., Warnken, W. & Izpisúa Belmonte, J. C. Dorsal cell fate specified by chick Lmx1 during vertebrate limb development. Nature 378, 716–720 (1995).
Loomis, C. A. et al. The mouse Engrailed-1 gene and ventral limb patterning. Nature 382, 360–363 (1996).
Niswander, L., Tickle, C., Vogel, A., Booth, I. & Martin, G. R. FGF-4-replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell 75, 579–587 (1993).
Niswander, L., Jeffrey, S., Martin, G. R. & Tickle, C. A. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature 371, 609–612 (1994).
Fallon, J. F. et al. FGF-2: Apical ectodermal ridge growth signal for chick limb development. Science 264, 104–107 (1994).
Ohuchi, H. et al. An additional limb can be induced form the flank of the chick embryo by FGF4. Biochem. Biophys. Res. Commun. 209, 809–816 (1995).
Cohn, M., Izpisúa Belmonte, J. C., Abud, H., Heath, J. K. & Tickle, C. FGF-2 application can induce additional limb formation from the flank of chick embryos. Cell 80, 739–746 (1995).
Laufer, E., Nelson, C. E., Johnson, R. L., Morgan, B. A. & Tabin, C. Sonic hedgehog and Fgf-4 act through a signalling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell 79, 993–1003 (1994).
Mahmood, R. et al. A role for FGF8 in the initiation and maintenance of vertebrate limb bud outgrowth. Curr. Biol. 5, 797–806 (1995).
Crossley, P. H., Minowada, G., MacArthur, C. A. & Martin, G. R. Roles for FGF-8 in the induction, initiation and maintenance of chick limb development. Cell 84, 127–136 (1996).
Vogel, A., Rodriguez, C. & Izpisúa Belmonte, J. C. Involvement of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Development 122, 1737–1750 (1996).
Irvine, K. D. & Wieschaus, E. Fringe, a boundary-specific signaling molecule, mediates interactions between dorsal and ventral cells during Drosophila wing development. Cell 79, 595–606 (1994).
Kim, J., Irvine, K. D. & Carroll, S. B. Cell recognition, signal induction, and symmetrical gene activation at the dorsal-ventral boundary of the developing Drosophila wing. Cell 82, 795–802 (1995).
Diaz-Benjumea, F. J. & Cohen, S. M. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121, 4215–4225 (1995).
Kim, J. et al. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382, 133–138 (1996).
Neumann, C. J. & Cohen, S. M. A hierarchy of cross-regulation involving Notch, wingless, vestigal and cut organizes the dorsal-ventral axis of the Drosophila wing. Development 122, 3477–3485 (1996).
De Celis, J. F., Garcia-Bellido, A. & Bray, S. J. Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development 122, 359–369 (1996).
Wu, J. Y., Wen, L., Zhang, W.-J. & Rao, Y. The secreted product of Xenopus gene lunatic fringe, a vertebrate signaling molecule. Science 273, 355–358 (1996).
Yuan, Y. P., Schultz, J., Mlodzik, M. & Bork, P. Secreted Fringe-like signalling molecules may be glycosyltransferases. Cell 88, 9–11 (1997).
Cope, L. D. et al. Molecular cloning of a gene involved in lipooligosaccharide biosynthesis and virulence expression by Haemophilus influenzae type B. Mol. Microbiol. 5, 1113–1124 (1991).
Hamburger, V. & Hamilton, H. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951).
Ros, M. A. et al. The limb field mesoderm determines initial limb bud anteroposterior asymmetry and budding independent of sonic hedgehog or apical ectodermal gene expressions. Development 122, 2319–2330 (1996).
Laufer, E. et al. Expression of Radical fringe in limb-bud ectoderm regulates apical ectodermal ridge formation. Nature 386, 366–373 (1997).
Couso, J. P., Kust, E. & Martinez Arias, A. Serrate and wingless in Drosophila wing development. Curr. Biol. 5, 1437–1448 (1995).
Goodrich, L. V., Johnson, R. L., Milenkovic, L., McMahon, J. A. & Scott, M. P. Conservation of the Hedgehog/patched/signaling pathway from flies to mice: Induction of a mouse patched gene by Hedgehog. Genes Dev. 10, 301–312 (1996).
Riddle, R. D., Johnson, R. L., Laufer, E. & Tabin, C. Sonic hedgehog mediates the polarizing activity of theZPA. Cell 75, 1401–1416 (1993).
Francis, P. H., Richardson, M. K., Brickell, P. M. & Tickle, C. Bone morphogenetic proteins and a signaling pathway that controls pattern in the developing chick limb. Development 120, 209–218 (1994).
Marigo, V., Scott, M. P., Johnson, R. L., Goodrich, L. V. & Tabin, C. J. Conservation in hedgehog signaling: induction of a chicken patched homolog by sonic hedgehog in the developing limb. Development 122, 1225–1233 (1996).
Zecca, M., Basler, K. & Struhl, G. Sequential organizing activities of engrailed hedgehog and decapentaplegic in the Drosophila wing. Development 121, 2265–2278 (1995).
Myat, A., Henrique, D., Ish-Horowicz, D. & Lewis, J. A chick homologue of serrate and its relationship with notch and delta homologues during central neurogenesis. Dev. Biol. 174, 233–247 (1996).
Shawber, C. J., Boulter, J. & Weinmaster, G. Jagged2: a serrafe-like gene expressed during rat embryogenesis. Dev. Biol. 180, 370–376 (1996).
Garcia-Bellido, A. How organisms are put together. Eur. Rev. 2, 15–21 (1994).
Izpisúa Belmonte, J. C., Falkenstein, H., Dolle, P., Renucci, A. & Duboule, D. Murine genes related to the Drosophila AbdB homeotic gene are sequentially expressed during development of the posterior part of the body. EMBO J. 10, 2279–2289 (1991).
Izpisúa Belmonte, J. C., De Robertis, E. M., Storey, K. G. & Stern, C. The homeobox gene goosecoid and the origin of organizer cells in the early chick blastoderm. Cell 74, 645–659 (1993).
Izpisúa Belmonte, J. C., Tickle, C., Dolle, P., Wolpert, L. & Duboule, D. Expression of the homeobox Hox-4 genes and the specification of position in chick wing development. Nature 350, 585–589 (1991).
Morgan, B. A., Izpisúa Belmonte, J. C., Duboule, D. & Tabin, C. J. Targeted misexpression of Hox-4.6 in the avian limb bud causes apparent homeotic transformations. Nature 358, 236–239 (1992).
Hughes, S., Greenhouse, J., Petropoulos, J. & Sutrave, P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J. Virol. 61, 3004–3012 (1987).
Gilbert, S. F. Dev. Biol. (Sinauer, Sunderland, MA, 1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rodriguez-Esteban, C., Schwabe, J., Peña, J. et al. Radical fringe positions the apical ectodermal ridge at the dorsoventral boundary of the vertebrate limb. Nature 386, 360–366 (1997). https://doi.org/10.1038/386360a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/386360a0
This article is cited by
-
Glycosylation regulates Notch signalling
Nature Reviews Molecular Cell Biology (2003)
-
Dual role of the fringe connection gene in both heparan sulphate and fringe-dependent signalling events
Nature Cell Biology (2001)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.