Abstract
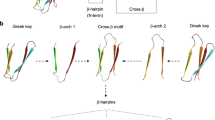
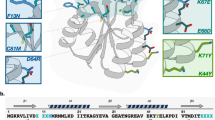
A MAJOR challenge in protein design is to create stable scaffolds into which tailored functions can be introduced. Here we present the design, synthesis and characterization of a 61-residue all-β protein: the minibody. We used a portion of the heavy chain variable domain of an immunoglobulin as a template, obtaining a molecule with a novel β-sheet scaffold and two regions corresponding to the hypervariable loops HI and H2. To exploit the potential for creating functional centres in the minibody, we engineered a metal-binding site into it. This site is formed by one histidine in HI and two in H2. The protein is folded, compact and able to bind metal, thus representing the first designed β-protein with a novel fold and a tailored function. By randomizing the sequence of the hypervariable loops, we are using the minibody scaffold to construct a conformationally constrained peptide library displayed on phage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Chothia, C. & Lesk, A. M. J. molec. Biol. 196, 901–917 (1987).
Bruccoleri, R. E., Haber, E. & Novotny, J. Nature 335, 564–568 (1988).
Martin, A. C. R., Cheetham, J. C. & Rees, A. R. Meth. Enzym. 203, 121–153 (1991).
Richardson, J. S. & Richardson, D. C. Trends biochem. Sci. 14, 304–309 (1989).
Hill, C. P., Anderson, D. H., Wesson, L., DeGrado, W. F. & Eisenberg, D. Science 249, 543–546 (1990).
Hecht, M. H., Richardson, J. S., Richardson, D. C. & Odgen, R. C. Science 249, 884–891 (1990).
Fedorov, A. N. et al. J. molec. Biol. 225, 927–931 (1992).
Bianchi, E., Sollazzo, M., Tramontano, A. & Pessi, A. Int. J. Peptide Protein Res. (in the press).
Kubiak, T., Whitney, D. B. & Merrifield, R. B. Biochemistry 26, 7849–7855 (1987).
Segal, D. M. et al. Proc. natn. Acad. U.S.A. 71, 4298–4302 (1974).
Regan, L. & Clarke, N. D. Biochemistry 29, 10878–10883 (1990).
Higaki, J. N., Haymore, B. L., Chen, S., Fletteriok, R. J. & Craik, C. S. Biochemistry 29, 8582–8586 (1990).
Iverson, B. L. et al. Science 249, 659–662 (1990).
Arnold, F. H. & Haymore, B. L. Science 25, 1796–1797 (1991).
Vallee, B. L. & Auld, D. S. Biochemistry 29, 5647–5656 (1990).
Bianchi, E., Sollazzo, M., Tramontano, A. & Pessi, A. Int. J. Peptide Protein Res. (in the press).
Goto, Y. & Hamagouchi, K. J. Biochem. 86, 1433–1441 (1979).
Pace, C. N. Meth. Enzym. 131, 266–280 (1986).
Lindskog, S. & Nyman, P. O. Biochim. biophys. Acta 85, 462–474 (1964).
Creighton, T. E. Biochem. J. 270, 1–16 (1990).
Smith, G. P. Curr. Opin. Biotechnol. 2, 668–673 (1991).
Saragovi, H. U., Greene, M. I., Chrusciel, R. A. & Kahn, M. Biotechnology 10, 773–778 (1992).
Kannan, K. K. et al. Proc. natn. Acad. Sci. U.S.A. 72, 51–55 (1975).
Chang, X. T., Wu, C. S. C. & Yang, J. Y. Analyt. Biochem. 91, 13–31 (1978).
Levitt, M. & Greer, J. J. molec. Biol. 114, 181–293 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pessi, A., Bianchi, E., Crameri, A. et al. A designed metal-binding protein with a novel fold. Nature 362, 367–369 (1993). https://doi.org/10.1038/362367a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/362367a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.