Abstract
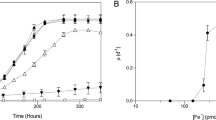
IN the oceans, many trace metals show a surface depletion relative to deep waters that is typical of the principal algal nutrients, N, ¡ and Si, and is therefore presumed to result from biological uptake at the sea surface and regeneration at depth. Among trace metals, cadmium has an especially acute surface depletion1,2, and shows the best correlation with a major algal nutrient (P) 1–3. But the biological reason for Cd surface depletion is particularly puzzling, because unlike other surface-depleted trace elements (for example, Fe, Ni, Co, Zn and Cu), Cd is not known to be required by organisms. However, because Cd can substitute for Zn in some metalloenzymes in vitro4 and in vivo5, we hypothesized that Cd might promote the growth of Zn-limited phytoplankton. Marine phytoplankton are limited by a free Zn ion activity of 10–11.5 M (refs 6, 7), which is similar to the activity estimated for ocean surface waters8 as a result of the low concentration and organic complexation of Zn in the oceans. We now report that, in sea water with low Zn concentration, mimicking conditions of the ocean surface waters, Cd stimulates the growth of the marine diatom Thalassiosira weissflogii by substituting for Zn in certain macromolecules. The substitution is highly effective, in that Zn-defÃcient cells can grow at 90% of their maximum rate when supplied with Cd. We also find that Co can substitute for Zn (although less efficiently than Cd), indicating that Co could be an important nutrient for algal growth for reasons other than its role in vitamin B12 (ref. 9).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Boyle, E., Sciater, F. R. & Edmond, J. M. Nature 263, 42–44 (1976).
Bruland, K. W., Knauer, G. A. & Martin, J. H. Limnol. Oceanogr. 23, 618–625 (1978).
Martin, J., Bruland, K. W. & Broenkow, W. in Marine Pollutant Transfer (eds Windom, H. L. & Duce, R. A.) 159–184 (Heath, Lexington, Massachusetts, 1976).
Bertini, I. & Luchinat, C. in Metal Ions in Biological Systems Vol. 15 (ed. Sigel, H.) 101–156 (Dekker, Basle, 1983).
Rosenbusch, J. P. & Weber, K. Proc. natn. Acad. Sci. U.S.A. 68, 1019–1023 (1971).
Anderson, M. A., Morel, F. M. M. & Guillard, R. R. L. Nature 276, 70–71 (1978).
Brand, L. E., Sunda, W. G. & Guillard, R. R. L. Limnol. Oceanogr. 28, 1182–1195 (1983).
Bruland, K. W. Limnol. Oceanogr. 34, 269–285 (1989).
Babior, B. M. (ed.) Cobalamin: Biochemistry and Pathophysiology (Wiley, New York, 1975).
Morel, F. M. M., Reuter, J. G., Anderson, D. M. & Guillard, R. R. L. J. Phycol. 15, 135–141 (1979).
Westall, J. C., Zachary, J. L. & Morel, F. M. M. MINEQL: A Computer Program for the Calculation of Chemical Equilibrium Composition of Aqueous Systems (Department of Civil Engineering, MIT. Cambridge, Massachusetts, 1976).
Ringbom, A. Complexation in Analytical Chemistry (Interscience, New York, 1963).
Brand, L. E., Guillard, R. R. L. & Murphy, L. S. J. Plankton Res. 3, 193–201 (1981).
Keller, M. D., Bellows, W. K. & Guillard, R. R. L. J. exp. mar. Biol. Ecol. 117, 279–283 (1988).
Lowry, O. H. et al. J. biol. Chem. 193, 265–275 (1951).
Graham, D., Reed, M. L., Patterson, B. D., Hockley, D. G. & Dwyer, M. R. in Biology and Chemistry of the Carbonic Anhydrases (eds Tashian, R. E. & Hewett-Emmett, D.) 222–237 (New York Academy of Sciences, New York, 1984).
Vallee, B. L. & Galdes, A. Adv. Enzym. 56, 283–430 (1984).
Perry, M. J. Mar. Biol. 15, 113–119 (1972).
Gekeler, W., Grill, E., Winnacker, E.-L. & Zenk, M. H. Arch. Microbiol. 150, 197–202 (1988).
Grill, E., Winnacker, E.-L. & Zenk, M. H. Proc. natn Acad. Sci. U.S.A. 84, 439–443 (1987).
Grill, E., Loffler, S., Winnacker, E.-L. & Zenk, M. H. Proc. natn. Acad. Sci. U.S.A. 86, 6838–6842 (1989).
Sherrell, R. M. thesis, MIT (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Price, N., Morel, F. Cadmium and cobalt substitution for zinc in a marine diatom. Nature 344, 658–660 (1990). https://doi.org/10.1038/344658a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/344658a0
This article is cited by
-
Adaptive responses of marine diatoms to zinc scarcity and ecological implications
Nature Communications (2022)
-
Dinoflagellates alter their carbon and nutrient metabolic strategies across environmental gradients in the central Pacific Ocean
Nature Microbiology (2021)
-
Shifts in photosynthetic parameters and lipid production of the freshwater microalga Selenastrum gracile (Chlorophyceae) under cadmium exposure
Journal of Applied Phycology (2020)
-
A new widespread subclass of carbonic anhydrase in marine phytoplankton
The ISME Journal (2019)
-
Emergence of metal selectivity and promiscuity in metalloenzymes
JBIC Journal of Biological Inorganic Chemistry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.