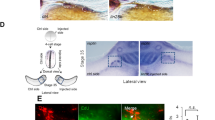
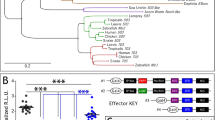
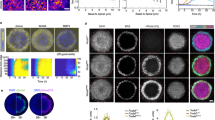
Abstract
ALL-trans retinoic acid (RA) is well known as a biologically active form of vitamin A and a teratogen1-8. The identification of nuclear receptors for this ligand9-11 suggests strongly that it is an endogenous signal molecule, and measurements of RA12 and teratogenic manipulations13-15 suggest further that RA is a mor-phogen specifying the anteroposterior axis during limb development. Besides the limb, RA and other retinoids affect development of other organs, including the central nervous system (CNS)4,6,7,16-18. None of these other effects has been investigated in detail. Our purpose here was to begin analysing the effects of RA on CNS development in Xenopus laevis. We find that RA acts on the developing CNS, transforming anterior neural tissue to a posterior neural specification. These and other19 findings raise the possibility that RA mediates an inductive interaction regulating anteroposterior differentiation within the CNS. Following recent reports implicating transforming growth factor-β2-like20,21 and fibroblast growth factor-like factors21,22 in mesoderm induction, this indicates that a different type of signal molecule (working through a nuclear receptor, not a plasma membrane receptor) might mediate inductive cell interactions during early embryonic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wolf, G., Physiol. Rev. 64, 3, 873–932 (1984).
Madani, K. A. Nutrition Res. 6, 107–123 (1986).
Chytil, F. J. Am. Acad. Dermat. 15, 741–747 (1986).
Yasuda, Y., Teratology 34, 37–49 (1986).
Sporn, M. B. & Roberts, A. B. (eds) Retinoids, Differentiation and Disease (CIBA Symposium 113, Pitman, London, 1985).
Shenfelt, R. E. Teratology 5, 1, 103–118 (1972).
Campbell, L. R., Hayton, D. H. & Sohal, G. S. Teratology 34, 171–197 (1986).
Fantel, A. G., Shephard, T. H., Newell-Morris, L. L. & Moffet, B. C. Teratology, 15, 65–72 (1977).
Petkovich, M., Brand, N. J., Krust, A. & Chambon, P. Nature 330, 444–450 (1987).
Giguere, V., Ong,, E. S., Segui, P. & Evans, R. M. Nature 330, 624–629 (1987).
Brand, N. et al. Nature 332, 850–853 (1988).
Thaller, C. & Eichele, G. Nature 327, 625–628 (1987).
Tickle, C., Alberts, B. M., Wolpert, L. & Lee, J. Nature 296, 564–565 (1982).
Summerbell, D. J. Embryol. exp. Morph. 78, 269–289 (1983).
Tickle, C., Lee, J. & Eichele, G. Devl Biol. 109, 82–95 (1985).
Lammer, E. J. et al. New Engl. J. Med. 313, 837–841 (1985).
Kochar, D. M. Acta path. microbiol. scand. 70, 398–404 (1967).
Morriss, G. M. J. Anat. 113, 241–250 (1972).
Dencker, L., d'Argy, R., Danielsson, B. R. G., Ghantous, H. & Sperter, G. O. Devl Pharmac. Ther. 10, 212–223 (1987).
Rosa, F. et al. Science 239, 783–785 (1988).
Kimmelman, D. & Kirschner, M. Cell 51, 869–877 (1987).
Slack, J. M. W., Darlington, B. G., Heath, J. K. & Godsave, S. F. Nature 326, 197–200 (1987).
McCormick, A. M. & Napoli, J. L. J. biol. Chem. 257, 1730–1735 (1982).
Eyal-Giladi, H. Extrait Arch. Biol. LXV. 2, 180–259 (1954).
Spemann, H. W. Wilhelm Roux Arch. Entw. Mech. Org. 123, 389–517 (1931).
Sala, M. Proc. K. ned. Acad. Wet. Series C58, 635–647 (1955).
Nieuwkoop, P. D. et al. J. exp. Zool. 120, 1–108 (1952).
Nieuwkoop, P. D. Adv. Morphogenesis 10, 2–39 (1973).
Toivonen, S. & Saxen, L. Science 159, 539–541 (1986).
Naspoa, J. W. & Kirschner, M. W. Cell 37, 731–742 (1984).
Eichele, G., Tickle, C. & Alberts, B. J. Cell Biol. 101, 1913–1920 (1985).
Flickinger, R. A. J. exp. Zool. 112, 165–185 (1949).
Woodland, H. R. & Jones, E. A. Development 101, 925–930 (1987).
McDevitt, D. S. & Brahma, S. K. J. exp. Zool. 186, 127–140 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Durston, A., Timmermans, J., Hage, W. et al. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature 340, 140–144 (1989). https://doi.org/10.1038/340140a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/340140a0
This article is cited by
-
Retinoic acid metabolism in cancer: potential feasibility of retinoic acid metabolism blocking therapy
Medical Molecular Morphology (2023)
-
Regulation of prefrontal patterning and connectivity by retinoic acid
Nature (2021)
-
All-trans retinoic acid stimulates the secretion of TGF-β2 via the phospholipase C but not the adenylyl cyclase signaling pathway in retinal pigment epithelium cells
BMC Ophthalmology (2019)
-
Parallel generation of easily selectable multiple nephronal cell types from human pluripotent stem cells
Cellular and Molecular Life Sciences (2019)
-
Hindbrain induction and patterning during early vertebrate development
Cellular and Molecular Life Sciences (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.