Abstract
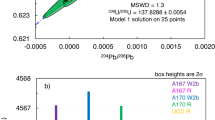
A group of 10 cubic diamonds from Zaire has been found1 to contain correlated concentrations of 40Ar and K which, interpreted as a whole-rock K–Ar isochron with the usual assumptions, yield the unreasonable age of 6.0 Gyr. The same age has also been determined2 by 40Ar–39Ar analysis of four additional diamonds from the same group. One explanation for these data is that the potassium in these diamonds is isotopically anomalous, specifically, enriched at least twofold in 40K relative to normal K, as might have resulted from preservation of cosmic-ray-induced spallation K produced in iron-rich planetesimals before accretion to the Earth. We have examined the isotopic composition of K in three diamonds from the same group, and report here that, within analytical uncertainties (about 1%), 40K is present in normal abundance, and the hypothesis of isotopically anomalous K is not supported. An alternative explanation is that the 40Ar in these diamonds is a trapped or 'excess' component, but this would require the unusual circumstance of a good correlation between 40Ar and K (for example, by incorporation of a fluid phase), but a lack of correlation between 40Ar and other noble gas species, including 36Ar.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Zashu, S., Ozima, M. & Nitoh, O. Nature 323, 710–712 (1986).
Takigami, Y., Ozima, M. & Zashu, S. Eos, Wash. 69, 503 (1988).
Imamura, M., Shima, M. & Honda, M. Z. Naturf 35A, 267–279 (1980).
Steiger, R. H. & Jager, E. Earth planet. Sci. Lett. 36, 359–362 (1977).
Garner, E. L., Murphy, T. J., Gramlich, J. W., Paulsen, P. J. & Barnes, I. L. J. Res. natn. Bur. Stand. 79A, 713–725 (1975).
Lewis, R. S., Tang, M., Wacker, J. F., Anders, E. & Steel, E., Nature 326, 160–162 (1987).
Funkhouser, J. G., Fisher, D. E. & Bonatti, E. Earth planet. Sci. Lett. 5, 95–100 (1968).
Allègre, C. J., Staudacher, T., Sarda, P. & Kurz, M. Nature 303, 762–766 (1983).
Ozima, M. & Podosek, F. Noble Gas Geochemistry (Cambridge University Press, 1983).
Fisher, D. E. Geochim. cosmochim. Acta 51, 2531–2541 (1987).
Ozima, M. & Zashu, S. Geochim. cosmochim. Acta 52, 19–25 (1988).
Ozima, M. A. rev. Earth planet. Sci. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Podosek, F., Pier, J., Nitoh, O. et al. Normal potassium, inherited argon in Zaire cubic diamonds. Nature 334, 607–609 (1988). https://doi.org/10.1038/334607a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/334607a0
This article is cited by
-
Diamond dating anomalies
Nature (1989)
-
Origin of the anomalous 40Ar—39Ar age of Zaire cubic diamonds: excess 40Ar in pristine mantle fluids
Nature (1989)
-
Isotopic and elemental evidence for a relationship between kimberlite and Zaire cubic diamonds
Nature (1988)
-
Mantle-derived fluids in diamond micro-inclusions
Nature (1988)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.