Abstract
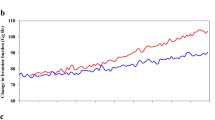
The importance of bromine chemistry was recognized shortly after Molina and Rowland1 had drawn attention to chlorine atom-catalysed destruction of ozone. Bromine atoms are even more efficient catalysts than chlorine2,3, and both may act synergistically to deplete ozone in the lower stratosphere4. Some measurements of bromine-containing source gases, such as CH3Br, CHBr3, CH2BrCH2Br, CH2Br2, CBrF3 and CBrClF2 have been reported for the troposphere5–8, their vertical distribution in the stratosphere is unknown except for CBrF3 (CFC-13B1) and CH3Br (ref. 6). These, together with the fully halogenated CBrClF2 (CFC-12B1), are probably the most important source gases for stratospheric bromine radicals. We report here the first stratospheric profiles of CBrClF2 up to 25 km, measured at 44° N during three consecutive years. From these data it follows that the atmospheric abundance of this halocarbon is growing rapidly, at a rate of 20% per year in the Northern Hemisphere, corresponding to a worldwide emission of ∼3.6×103 tons per year.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Molina, J. & Rowland, F. S. Nature 249, 810–815 (1974).
Watson, R. T. The Natural Stratosphere of 1974 (CIAP Monogr. 1, Sect. 5.7.5, Department of Transportation, Washington DC, 1975).
Wofsy, S. C. et al. Geophys. Res. Lett. 2, 215–218 (1975).
Yung, Y. L. et al. J. atmos. Sci. 37, 339–353 (1980).
Singh, H. B. et al. J. geophys. Res. 88, 3675–3683 (1983).
Fabian, P. et al. Nature 294, 733–735 (1981).
Berg, W. W. et al. Geophys. Res. Lett. 11, 429–432 (1984).
Rasmussen, R. A. & Khalil, M. A. K. Geophys. Res. Lett. 11, 433–436 (1984).
Molina, L. T. et al. J. phys. Chem. 86, 2672–2676 (1982).
Penkett, S. A. et al. J. geophys. Res. 86, 5172–5178 (1981).
Fabian, P. et al. J. geophys. Res. 84, 3149–3154 (1979).
Fabian, P. & Gömer, D. Fresenius Z. Analyt. Chem. 319, 890–897 (1984).
Fabian, P. et al. J. geophys. Res. 86, 5179–5184 (1981).
Schmidt, M. J geophys. Res. 87, 11239 (1982).
Lal, S. et al. Proc. int. Ozone Symp. (eds Zerefos, C. S. & Ghazi, A.) 134 (Reidel, Dordrecht, 1985).
Bichof, W. et al. Nature (in the press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lal, S., Borchers, R., Fabian, P. et al. Increasing abundance of CBrClF2 in the atmosphere. Nature 316, 135–136 (1985). https://doi.org/10.1038/316135a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/316135a0
This article is cited by
-
A decrease in the growth rates of atmospheric halon concentrations
Nature (1992)
-
Ultraviolet absorption spectrum of trifluoro-bromo-methane, difluoro-dibromo-methane and difluoro-bromo-chloro-methane in the vapor phase
Journal of Atmospheric Chemistry (1989)
-
Measurements of atmospheric BrOx radicals in the tropical and mid-latitude atmosphere
Nature (1988)
-
Scenarios of possible changes in atmospheric temperatures and ozone concentrations due to man's activities, estimated with a one-dimensional coupled photochemical climate model
Climate Dynamics (1988)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.