Abstract
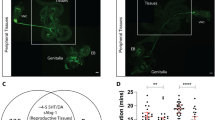
Observations in insects reveal sexual differences in the central nervous system (CNS), associated with sexually dimorphic patterns of reproductive behaviour1–9. For example, in certain species of moths, including the sphinx moth Manduca sexta, only males fly towards a sexually receptive female or towards a source of the female sex pheromone10,11. In Manduca, specialized olfactory receptor cells found only on male antennae12,13 respond sensitively and selectively to the female sex pheromone (unpublished experiments with K.-E. Kaissling and R. J. O'Connell). Their axons project into the macroglomerular complex (MGC), which is characteristic of male, but not female, antennal lobes (ALs; Fig. 1b, d)1–3,5,8,9,14. These afferents to the MGC presumably synapse with male-specific AL neurones8,15 to begin the processing of phenomonal information. We have now devised a surgical procedure for producing antennal gynandromorphs of Manduca in which one of the two ALs receives sensory innervation from an antenna formed by a transplanted imaginal disk of the opposite sex. We report here that in these gynandromorphs, the physiological and morphological properties of certain AL neurones are influenced by the gender of the antennal sensory axons contacting them. In particular, neurones resembling the male-specific AL neurones appear in female ALs innervated by sensory axons from a grafted male antenna.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Boeckh, J., Boeckh, V. & Kuhn, A. Olfaction Taste, Paris 6, 315–321 (1977).
Boeckh, J. & Boeckh, V. J. comp. Physiol. 132, 235–242 (1979).
Camazine, S. & Hildebrand, J. Soc. Neurosci. Abstr. 5, 155 (1979).
Hausen, K. & Strausfeld, N. J. Proc. R. Soc. B208, 57–71 (1980).
Hildebrand, J. G. et al. in Insect Neurobiology and Pesticide Action (ed. Rickett, F. E.) 375–382 (Society for Chemical Industry, London, 1980).
Strausfeld, N. J. Nature 283, 381–383 (1980).
Wohlers, D. & Bacon, J. Cell Tissue Res. 209, 371–382 (1980).
Matsumoto, S. G. & Hildebrand, J. G. Proc. R. Soc. B213, 249–277 (1981).
Prillinger, L. Cell Tissue Res. 215, 563–575 (1981).
Allen, N. & Hodge, C. R. J. econ. Ent. 48, 526–528 (1955).
Allen, N., Kinard, W. S. & Jacobson, M. J. econ. Ent. 55, 347–351 (1962).
Sanes, J. R. & Hildebrand, J. G. Devl Biol. 51, 300–319 (1976).
Sanes, J. R. & Hildebrand, J. G. Devl Biol. 51, 282–299 (1976).
Jawlowski, H. Annls Univ. Mariae Curie, Sklodowska C8, 403–434 (1954).
Tolbert, L. P. & Hildebrand, J. G. Proc. R. Soc. B213, 279–301 (1981).
Sanes, J. R. & Hildebrand, J. G. Wilhelm Roux's Arch. devl Biol. 178, 71–78 (1975).
Sanes, J. R., Prescott, D. J. & Hildebrand, J. G. Brain Res. 119, 389–402 (1977).
Hildebrand, J. G., Hall, L. M. & Osmond, B. C. Proc. natn. Acad. Sci. U.S.A. 76, 499–503 (1979).
Schneiderman, H. A. in Methods in Developmental Biology (eds Wilt, F. H. & Wessels, N. K.) 753–766 (Crowell, New York, 1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schneiderman, A., Matsumoto, S. & Hildebrand, J. Trans-sexually grafted antennae influence development of sexually dimorphic neurones in moth brain. Nature 298, 844–846 (1982). https://doi.org/10.1038/298844a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/298844a0
This article is cited by
-
Mixture and odorant processing in the olfactory systems of insects: a comparative perspective
Journal of Comparative Physiology A (2013)
-
Antagonistic roles of Wnt5 and the Drl receptor in patterning the Drosophila antennal lobe
Nature Neuroscience (2007)
-
Olfaction in Lepidoptera
Experientia (1995)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.