Volume 2
-
No. 12 December 2019
Branching through ring contractionThe branched pentose sugar d-apiose forms borate ester-polysaccharide crosslinks essential for plant cell wall development. Here the authors elucidate the multi-step reaction mechanism of UDP-apiose/UDP-xylose synthase involving decarboxylation of the UDP-d-glucuronic acid precursor coupled to pyranosyl-to-furanosyl sugar ring contraction by using the enzyme crystal structures and computational simulations.
See Savino et al.
-
No. 11 November 2019
Two eyes on single particlesWeckhuysen and co-workers report a set of catalyst sensors that allow for the simultaneous detection of local temperature and surface species on catalyst particles. This provides a powerful method to monitor, characterize and understand catalytic systems.
See Hartman et al.
-
No. 10 October 2019
Coated transition statesBy encapsulating palladium nanocrystals within microporous polymer layers of tunable composition, Cargnello and co-workers show an effective approach to control the catalytic activity of metal sites within a composite material. In the case of CO oxidation, the functionalities of the encapsulating layer exert a crucial function in governing the evolution of the transition state and regulating the diffusion of the product away from the active centre.
See Riscoe et al.
-
No. 9 September 2019
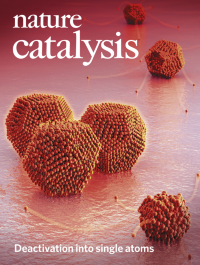
Deactivation into single atomsBy controlling the loading of metal nanoparticles in supported catalysts using colloidal nanocrystals, Cargnello and co-workers report a deactivation mechanism in combustion catalysts that occurs by particle decomposition into inactive single atoms rather than by particle growth. This deactivation process is fast, but is mitigated by the use of higher metal loadings, showing that, for certain reactions, higher particle densities lead to more stable catalysts.
See Goodman et al.
-
No. 8 August 2019
High five for oxygen reductionMetal-free nitrogen-doped carbon materials are promising catalysts for oxygen reduction, and have been successfully implemented in laboratory-scale polymer-electrolyte-membrane fuel cells and zinc-air batteries. Despite their widespread use, controversy still exists around what sites are the most active, although it is generally believed that these involve a nitrogen atom. Now, Yao, Dai and colleagues present evidence that pentagon defects are the main active sites of carbon materials for acidic oxygen reduction. The researchers do so by combining work-function analyses with macro/micro-electrochemical measurements on model highly oriented pyrolytic graphite with and without nitrogen doping.
See Jia et al.
-
No. 7 July 2019
Catalysis for transportationThe transportation sector represents a major area of research for the catalysis community. This Insight provides an overview of this prominent field, covering the topics of emissions control, production of hydrocarbon fuels and fuel cell powered engines.
The cover image comes from a Review Article on single-atom and few-atom cluster catalysts for CO oxidation by Atsushi Beniya and Shougo Higashi.
-
No. 6 June 2019
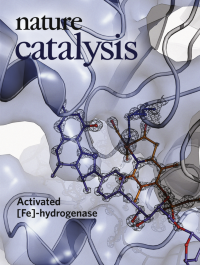
Activated [Fe]-hydrogenaseDetailed structural characterization of [Fe]-hydrogenase was so far limited to inactive states. Here, Huang et al. report the X-ray structure of [Fe]-hydrogenase in an active conformation. Based on this structure, computational simulations provide precise insights into H2-activation. This knowledge is important for exploiting [Fe]-hydrogenase for technological purposes such as hydrogenation reactions, production of H2 or using H2 as a fuel.
See Huang et al.
-
No. 5 May 2019
Accessing acetateUpgrading carbon dioxide-derived carbon monoxide to high-value chemicals is a potential route for carbon utilization. Here, Luc et al. report a Cu nanosheet catalyst that can convert carbon monoxide to acetate with a Faradaic efficiency of 48% at commercially relevant rates of reaction. The enhanced acetate selectivity is due to the suppression of the formation of ethylene and ethanol.
See Luc et al.
-
No. 4 April 2019
Ruthenium loners oxidise waterRu-based electrocatalysts are among the most active for the oxygen evolution reaction in acidic electrolyte but they commonly suffer from low stability. Here Wu, Li and co-workers report a core–shell catalyst consisting of a Pt3Cu core with a Pt-rich shell that stabilizes surface-dispersed Ru atoms. The compressive strain of the Pt shell fine-tunes the electronic structure of Ru sites, which results in high activity and stability for acidic water oxidation.
See Yao et al.
-
No. 3 March 2019
Synthetic lubricants from ethyleneWhile group 4 catalysts produce high-molecular-mass polyethylenes with spectacular efficiency, these catalysts are ineffective in producing low-molecular-mass highly-branched polyethylenes. Here Lohr, Marks and co-workers report the synthesis of these materials from abundant ethylene. The excellent activity and branch selectivity reflect previously unrecognized aspects of the cationic catalyst–counteranion pairing in nonpolar media. The products are rheologically and tribologically attractive candidates for synthetic lubricants.
See Gao et al.
-
No. 2 February 2019
Shifting reactivity pathwaysDue to electronic effects, certain activating groups in organic molecules can increase the reactivity of nearby bonds. Now, Lichosyt et al. have shown that such activating groups can be transiently introduced into otherwise unreactive molecules by catalytic reversible reactions. When combined with subsequent catalytic functionalization reactions, the constructed networks of reactions enable the simple functionalization of normally unreactive sites.
See Lichosyt et al.
-
No. 1 January 2019
Mapping bio-based chemicalsLee and co-workers discuss metabolically engineered microbial cells to produce chemicals of interest from renewable feedstocks. Biological routes in combination with chemical routes are presented in a bio-based chemicals map — serving as a blueprint for the future design of chemical biosynthesis strategies.
See Lee et al.