Abstract
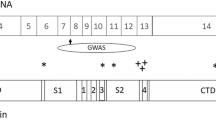
Metabotropic glutamate receptors (mGluRs) belong to the class of GTP-binding protein coupled receptors and consist of eight different subtypes. The subtype 2 metabotropic glutamate receptor (mGluR2) gene (GRM2) is one of the possible candidate genes for schizophrenia. Phencyclidine (PCP)-induced increase in glutamate efflux and schizophrenia-like behavioral abnormalities were reduced by pretreatment of the mGluRII agonist LY354740 in rats and its effects are mediated via mGluR2. To evaluate involvement of the mGluR2 gene in the pathogenesis of schizophrenia, we isolated the human mGluR2 gene and determined the transcription initiation site, the entire nucleotide sequence and the chromosomal localization. The hmGluR2 gene spans 13 kb with six exons, including one non-coding exon. The gene was mapped to chromosome 3 p12-p11 by Radiation Hybrid Panel analysis. We screened polymorphisms in the coding exons of the mGluR2 gene, using the SSCP procedure. The thirteen polymorphisms identified included ten missense, one silent mutation and two one-base substitutions in the 5′-untranslated region. We genotyped 213 Japanese schizophrenics and 220 controls to study the association of polymorphisms in the mGluR2 gene with schizophrenia. As we found no statistically significant differences in allele frequencies of each polymorphism, these polymorphisms apparently do not play a major role in schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nakanishi S . Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity Neuron 1994 13: 1031–1037
Nakanishi S, Masu M, Bessho Y, Nakajima Y, Hayashi Y, Shigemoto R . Molecular diversity of glutamate receptors and their physiological functions EXS 1994 71: 71–80
Holscher C, Gigg J, O'Mara SM . Metabotropic glutamate receptor activation and blockade: their role in long-term potentiation, learning and neurotoxicity Neurosci Biobehav Rev 1999 23: 399–410
Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination Science 2000 288: 1832–1835
Nicoletti F, Bruno V, Catania MV, Battaglia G, Copani A, Barbagallo G et al. Group-I metabotropic glutamate receptors: hypotheses to explain their dual role in neurotoxicity and neuroprotection Neuropharmacology 1999 38: 1477–1484
Crow TJ . Constrains on concepts of pathogenesis Arch Gen Psychiatry 1995 52: 1011–1014
Meador-Woodruff JH, Healy DJ . Glutamate receptor expression in schizophrenic brain Brain Res Rev 2000 31: 288–294
Olney JW, Farber NB . Glutamate receptor dysfunction and schizophrenia Arch Gen Psychiatry 1995 52: 998–1007
Javitt DC, Zukin SR . Recent advances in the phencyclidine model of schizophrenia Am J Psychiatry 1991 148: 1301–1308
Hoover F, Hankin MH, Radel JD, Reese JS, Goldman D . Axon-target interactions maintain synaptic gene expression in retinae transplanted to intracranial regions of the rat Brain Res Mol Brain Res 1997 51: 123–132
Hickmott PW, Constantine-Paton M . Experimental down-regulation of the NMDA channel associated with synapse pruning J Neurophysiol 1997 78: 1096–1107
Mohn AR, Gainetdinov RR, Caron MG, Koller BH . Mice with reduced NMDA receptor expression display behaviors related to schizophrenia Cell 1999 98: 427–436
Pin JP, Duvoisin R . The metabotropic glutamate receptors: structure and functions Neuropharmacology 1995 34: 1–26
Adams B, Moghaddam B . Corticolimbic dopamin neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine J Neurosci 1998 18: 5545–5554
Moghaddam B, Adams BW . Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats Science 1998 281: 1349–1352
Spooren WP, Gasparini F, van der Putten H, Koller M, Nakanishi S, Kuhn R . Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice Eur J Pharmacol 2000 397: R1–R2
Seidman JG . Screening of recombinant DNA libraries. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA et al (eds) Current Protocols in Molecular Biology John Wiley & Sons 1997 6.0.1–6.5.2
Hayashi K, Kukita Y, Inazuka M, Tahira T . Single-strand conformation polymorphism analysis. In: Cotton RGH, Edkins E, Forrest S (eds) Mutation Detection Oxford University Press: New York 1998 7–24
Xie X, Ott J . Testing linkage disequilibrium between a disease gene and marker loci Am J Hum Genet 1993 53: 1107
Adams MD, Kerlavage AR, Fleischmann RD, Fuldner RA, Bult CJ, Lee NH et al. Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence Nature 1995 377: 3–174
Flor PJ, Lindauer K, Puttner I, Ruegg D, Lukic S, Knopfel T et al. Molecular cloning, functional expression and pharmacological characterization of the human metabotropic glutamate receptor type 2 Eur J Neurosci 1995 7: 622–629
Riley BP, McGuffin P . Linkage and associated studies of schizophrenia Am J Med Genet 2000 97: 23–44
Minoshima T, Nakanishi S . Structural organization of the mouse metabotropic glutamate receptor subtype 3 gene and its regulation by growth factors in cultured cortical astrocytes J Biochem 1999 126: 889–896
Richardson-Burns SM, Haroutunian V, Davis KL, Watson SJ, Meador-Woodruff JH . Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus Biol Psychiatry 2000 47: 22–28
Hampson DR, Huang X, Pekhletski R, Peltekova V, Hornby G, Thomsen C et al. Probing the ligand-binding domain of the mGluR4 subtype of metabotropic glutamate receptor J Biol Chem 1999 274: 33488–33495
Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S . A family of metabotropic glutamate receptors Neuron 1992 8: 169–179
Li WH, Sadler LA . Low nucleotide diversity in man Genetics 1991 129: 513–523
Brookes AJ . The essense of SNPs Gene 1999 234: 177–186
Acknowledgements
We are grateful to all the medical staff who were involved in collecting specimens. We also thank Dr K Hayashi, Kyushu University for technical advice and Ms M Ohara for language assistance. This work was supported in part by Grant-in-Aid for Scientific Research on Priority Areas (c) Medical Genome Science from the Ministry of Education, Science, Sports and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joo, A., Shibata, H., Ninomiya, H. et al. Structure and polymorphisms of the human metabotropic glutamate receptor type 2 gene (GRM2): analysis of association with schizophrenia. Mol Psychiatry 6, 186–192 (2001). https://doi.org/10.1038/sj.mp.4000841
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4000841
Keywords
This article is cited by
-
Targeting metabotropic glutamate receptors for novel treatments of schizophrenia
Molecular Brain (2017)
-
In the grey zone between epilepsy and schizophrenia: alterations in group II metabotropic glutamate receptors
Acta Neurologica Belgica (2015)
-
Methamphetamine-Associated Psychosis
Journal of Neuroimmune Pharmacology (2012)
-
Group II metabotropic glutamate receptors and schizophrenia
Cellular and Molecular Life Sciences (2009)
-
The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity
Psychopharmacology (2008)