Abstract
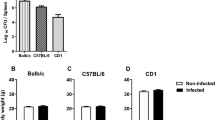
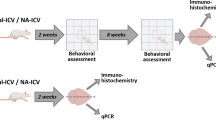
Epidemiological studies have indicated a link between certain neuropsychiatric diseases and exposure to viral infections. In order to examine long-term effects on behavior and gene expression in the brain of one candidate virus, we have used a model involving olfactory bulb injection of the neuro-adapted influenza A virus strain, WSN/33, in C57Bl/6 mice. Following this olfactory route of invasion, the virus targets neurons in the medial habenular, midline thalamic and hypothalamic nuclei as well as monoaminergic neurons in the brainstem. The mice survive and the viral infection is cleared from the brain within 12 days. When tested 14–20 weeks after infection, the mice displayed decreased anxiety in the elevated plus-maze and impaired spatial learning in the Morris water maze test. Elevated transcriptional activity of two genes encoding synaptic regulatory proteins, regulator of G-protein signaling 4 and calcium/calmodulin-dependent protein kinase IIα, was found in the amygdala, hypothalamus and cerebellum. It is of particular interest that the gene encoding RGS4, which has been related to schizophrenia, showed the most pronounced alteration. This study indicates that a transient influenza virus infection can cause persistent changes in emotional and cognitive functions as well as alterations in the expression of genes involved in the regulation of synaptic activities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mohammed AH, Norrby E, Kristensson K . Viruses and behavioural changes: a review of clinical and experimental findings. Rev Neurosci 1993; 4: 267–286.
Jordan I, Lipkin WI . Borna disease virus. Rev Med Virol 2001; 11: 37–57.
Conn CA, McClellan JL, Maassab HF, Smitka CW, Majde JA, Kluger MJ . Cytokines and the acute phase response to influenza virus in mice. Am J Physiol 1995; 268: R78–R84.
Fang J, Sanborn CK, Renegar KB, Majde JA, Krueger JM . Influenza viral infections enhance sleep in mice. Proc Soc Exp Biol Med 1995; 210: 242–252.
Swiergiel AH, Smagin GN, Johnson LJ, Dunn AJ . The role of cytokines in the behavioral responses to endotoxin and influenza virus infection in mice: effects of acute and chronic administration of the interleukin-1-receptor antagonist (IL-1ra). Brain Res 1997; 776: 96–104.
Brot MD, Rall GF, Oldstone MB, Koob GF, Gold LH . Deficits in discriminated learning remain despite clearance of long-term persistent viral infection in mice. J Neurovirol 1997; 3: 265–273.
Rubin SA, Sylves P, Vogel M, Pletnikov M, Moran TH, Schwartz GJ et al. Borna disease virus-induced hippocampal dentate gyrus damage is associated with spatial learning and memory deficits. Brain Res Bull 1999; 48: 23–30.
Rothschild DM, O'Grady M, Wecker L . Neonatal cytomegalovirus exposure decreases prepulse inhibition in adult rats: implications for schizophrenia. J Neurosci Res 1999; 57: 429–434.
Engel JA, Zhang J, Bergstrom T, Conradi N, Forkstam C, Liljeroth A et al. Neonatal herpes simplex virus type 1 brain infection affects the development of sensorimotor gating in rats. Brain Res 2000; 863: 233–240.
Mori I, Diehl AD, Chauhan A, Ljunggren HG, Kristensson K . Selective targeting of habenular, thalamic midline and monoaminergic brainstem neurons by neurotropic influenza A virus in mice. J Neurovirol 1999; 5: 355–362.
Smith W, Andrewes CH, Laidlaw PP . A virus obtained from influenza patients. Lancet 1933; 1: 66–68.
Stuart-Harris CH . A neurotropic strain of human influenza virus. Lancet 1939; 1: 497–499.
Francis T, Moore A . A study of the neurotropic tendency in strains of the virus of epidemic influenza. J Exp Med 1940; 72: 717–728.
Takahashi M, Yamada T, Nakajima S, Nakajima K, Yamamoto T, Okada H . The substantia nigra is a major target for neurovirulent influenza A virus. J Exp Med 1995; 181: 2161–2169.
Reinacher M, Bonin J, Narayan O, Scholtissek C . Pathogenesis of neurovirulent influenza A virus infection in mice. Route of entry of virus into brain determines infection of different populations of cells. Lab Invest 1983; 49: 686–692.
Park CH, Ishinaka M, Takada A, Kida H, Kimura T, Ochiai K et al. The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch Virol 2002; 147: 1425–1436.
Aronsson F, Robertson B, Ljunggren HG, Kristensson K . Invasion and persistence of the neuroadapted influenza virus A/WSN/33 in the mouse olfactory system. Viral Immunol 2003; 16: 415–423.
Aronsson F, Karlsson H, Ljunggren HG, Kristensson K . Persistence of the influenza A/WSN/33 virus RNA at midbrain levels of immunodefective mice. J Neurovirol 2001; 7: 117–124.
Maier W, Zobel A, Rietschel M . Genetics of schizophrenia and affective disorders. Pharmacopsychiatry 2003; 36: S195–S202.
Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P . Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron 2000; 28: 53–67.
Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P . Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry 2001; 6: 293–301.
Chowdari KV, Mirnics K, Semwal P, Wood J, Lawrence E, Bhatia T et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet 2002; 11: 1373–1380.
Morris DWRA, McGhee KA, Schwaiger S, Scully P, Quinn J, Meagher D et al. Confirming RGS4 as a susceptibility gene for schizophrenia. Am J Med Genet 2004; 125: 50–53.
Tobita K, Sugiura A, Enomote C, Furuyama M . Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol (Berl) 1975; 162: 9–14.
Kuzmin A, Sandin J, Terenius L, Ogren SO . Dose- and time-dependent bimodal effects of kappa-opioid agonists on locomotor activity in mice. J Pharmacol Exp Ther 2000; 295: 1031–1042.
Ogren SO, Hall H, Kohler C, Magnusson O, Sjostrand SE . The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine-mediated motor functions. Psychopharmacology (Berl) 1986; 90: 287–294.
Ogren SO, Kehr J, Schott PA . Effects of ventral hippocampal galanin on spatial learning and on in vivo acetylcholine release in the rat. Neuroscience 1996; 75: 1127–1140.
Misane I, Ogren SO . Multiple 5-HT receptors in passive avoidance: comparative studies of p-chloroamphetamine and 8-OH-DPAT. Neuropsychopharmacology 2000; 22: 168–190.
Franklin K, Paxinos G . The Mouse Atlas in Stereotaxic Coordinates. Academic Press: San Diego, CA, 1997.
Livak KJ ST . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Wiens BL . A fixed sequence Bonferroni procedure for testing multiple endpoints. Pharm Statist 2003; 2: 211–215.
Ingi T, Aoki Y . Expression of RGS2, RGS4 and RGS7 in the developing postnatal brain. Eur J Neurosci 2002; 15: 929–936.
Hong JX, Wilson GL, Fox CH, Kehrl JH . Isolation and characterization of a novel B cell activation gene. J Immunol 1993; 150: 3895–3904.
Murphy JJ, Norton JD . Cell-type-specific early response gene expression during plasmacytoid differentiation of human B lymphocytic leukemia cells. Biochim Biophys Acta 1990; 1049: 261–271.
Siderovski DP, Heximer SP, Forsdyke DR . A human gene encoding a putative basic helix–loop–helix phosphoprotein whose mRNA increases rapidly in cycloheximide-treated blood mononuclear cells. DNA Cell Biol 1994; 13: 125–147.
Bejar R, Yasuda R, Krugers H, Hood K, Mayford M . Transgenic calmodulin-dependent protein kinase II activation: dose-dependent effects on synaptic plasticity, learning, and memory. J Neurosci 2002; 22: 5719–5726.
Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H . Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 2001; 411: 801–805.
Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ . Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature 2001; 411: 309–313.
Molnar M, Potkin SG, Bunney WE, Jones EG . mRNA expression patterns and distribution of white matter neurons in dorsolateral prefrontal cortex of depressed patients differ from those in schizophrenia patients. Biol Psychiatry 2003; 53: 39–47.
Sieck MH, Gordon BL . Selective olfactory bulb lesions: reactivity changes and avoidance learning in rats. Physiol Behav 1972; 9: 545–552.
Primeaux SD, Holmes PV . Role of aversively motivated behavior in the olfactory bulbectomy syndrome. Physiol Behav 1999; 67: 41–47.
Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B . Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Res 1992; 587: 181–185.
Kelly JP, Wrynn AS, Leonard BE . The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther 1997; 74: 299–316.
Morris RG, Garrud P, Rawlins JN, O'Keefe J . Place navigation impaired in rats with hippocampal lesions. Nature 1982; 297: 681–683.
LeDoux JE . Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155–184.
Fendt M, Fanselow MS . The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev 1999; 23: 743–760.
Ellison G . Stimulant-induced psychosis, the dopamine theory of schizophrenia, and the habenula. Brain Res Brain Res Rev 1994; 19: 223–239.
Pierson R, Corson PW, Sears LL, Alicata D, Magnotta V, Oleary D et al. Manual and semiautomated measurement of cerebellar subregions on MR images. Neuroimage 2002; 17: 61–76.
Acknowledgements
This study was supported by grants from the Stanley Medical Research Institute, the Swedish Research Council (04480 and 11588) and Stiftelserna Sigurd och Elsa Goljes minne, Ragnhild och Einar Lundströms minne, AlzheimerFonden and Wallenberg Consortium North. F Aronsson is the recipient of a scholarship from the ‘Network for Inflammation Research’ funded by the Swedish Foundation for Strategic Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beraki, S., Aronsson, F., Karlsson, H. et al. Influenza A virus infection causes alterations in expression of synaptic regulatory genes combined with changes in cognitive and emotional behaviors in mice. Mol Psychiatry 10, 299–308 (2005). https://doi.org/10.1038/sj.mp.4001545
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001545
Keywords
This article is cited by
-
Cognitive decline following acute viral infections: literature review and projections for post-COVID-19
European Archives of Psychiatry and Clinical Neuroscience (2022)
-
Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients
Neurological Sciences (2020)
-
Influenza A virus-dependent remodeling of pulmonary clock function in a mouse model of COPD
Scientific Reports (2015)
-
Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees
Scientific Reports (2015)
-
Decreased Na+ influx lowers hippocampal neuronal excitability in a mouse model of neonatal influenza infection
Scientific Reports (2015)