Abstract
The association of Epstein–Barr virus with pulmonary neoplasms has been restricted to lymphoepithelioma-like carcinomas in Asian patients. We have selected 19 pulmonary adenocarcinomas and squamous-cell carcinomas from 1545 pulmonary neoplasms diagnosed from 1996 to 2007 in an occidental population. All of them showed a low-power appearance confusing between an epithelial and a lymphoid neoplasm, with a dense lymphocytic infiltrate intermingled with neoplastic cells giving an image akin to lymphoepithelial complexes. Five carcinomas presented typical features of Lymphoepithelioma-like lung carcinomas; but six cases could be classified as squamous-cell carcinomas and eight as adenocarcinomas. A semiquantitative polymerase chain reaction method, Early RNA genes 1 and 2 in situ hybridization as well as Latent membrane protein immunostaining for Epstein–Barr virus DNA, RNA and protein detection methods were used in every case. None of Lymphoepithelioma-like carcinomas showed positivity for Epstein–Barr virus in any used method. Otherwise four squamous-cell carcinomas and eight adenocarcinomas (12 cases) demonstrated viral sequences in polymerase chain reaction and/or in situ hybridization analysis in neoplastic cells. Moreover two adenocarcinomas also displayed human herpesvirus 6 DNA sequences coamplification in molecular analysis. Protein immunostaining was focally positive in only three cases. We performed the same analysis in 70 more cases of conventional pulmonary squamous-cell carcinomas and adenocarcinomas that gave negative results. In conclusion, a subset of pulmonary squamous-cell carcinomas and adenocarcinomas show Epstein–Barr DNA and/or RNA sequences in neoplastic cells. This finding expands the spectra of epithelial cell common tumours Epstein–Barr virus associated.
Similar content being viewed by others
Main
Lung cancer is one of the most usual human solid tumours with known aetiology. In fact, 85–90% of the cases are associated with cigarette smoking. There are, however, some environmental agents, such as exposures to radon1 silica or asbestos that have also been implicated in its development.2
Among the environmental carcinogenetic agents, some viruses have been closely linked with many human cancers. The Epstein–Barr virus (EBV) was the first to be accepted as a human tumour virus associated with Burkitt's lymphoma,3 and since then, EBV has been correlated with other specific malignancies as Hodgkin's disease, B-cell lymphomas in immunosuppressed individuals and a variety of undifferentiated carcinomas (the so-called lymphoepitheliomas), especially those occurring in the nasopharynx and foregut-derived organs.4 In the case of the lung, the last WHO classification of pulmonary neoplasms has classified lymphoepithelioma-like carcinoma as a large-cell carcinoma variant. It is defined as an undifferentiated carcinoma that shows a typical syncytial growth pattern of undifferentiated neoplastic epithelial cells, with vesicular nuclei and prominent nucleoli, similar to the nasopharynx counterpart.5
Primary lymphoepithelioma-like carcinoma of the lung is very rare and most of reports come from Chinese or Taiwanese patients,6, 7, 8 and to date the association of EBV with these kind of lung carcinomas is restricted to Asian patients9, 10 (Table 1). On the other hand, the presence of EBV DNA has been described occasionally in pulmonary carcinomas other than lymphoepithelioma-like in Asian patients,11, 17, 16 and in two Caucasian patients.18, 19 In consequence, it seems that EBV-associated lung carcinomas different to the typical lymphoepithelioma-like type could exist.17, 16
Our aim is to describe a series of pulmonary adenocarcinomas and squamous-cell carcinomas found in a 12-year period, characterized by a dense lymphoid stroma in an occidental population, and to demonstrate the presence of EBV in this kind of carcinomas.
Materials and methods
All the pulmonary carcinomas not classified as small-cell carcinomas, surgically removed and diagnosed in the Pathology Department of the University Hospital Marques de Valdecilla from 1996 to December 2007, were reviewed by two independent pathologists (JJGR and JFVB). From 1545 cases, we selected 19 cases that showed a prominent lymphoid stroma, with indistinct limits between neoplastic groups and tumoral stroma. Characteristically the low-power appearance was confusing between an epithelial and a lymphoid neoplasm. We reviewed the clinical charts in every case to check clinicopathologic stage, serology for EBV, if available, and survival data.
A total of 70 cases of otherwise conventional pulmonary squamous-cell carcinomas and adenocarcinomas were selected from our surgical pathology files. All paraffin-embedded donor tissue blocks were sampled with 0.6-mm punchers using a Beecher tissue microarray instrument (Beecher Instruments Inc. Sun Prairie, WI, USA). Paraffin tissue array blocks containing arrayed core cylinders from those 70 cases were subjected to routine hematoxylin and eosin staining, in situ hybridization (ISH) and LMP1 immunostaining.
At the same time, we selected 20 cases of squamous and adenocarcinomas that were subjected to molecular analysis for viral sequences by PCR methods.
Molecular Analysis for Viral Sequences
In the 19 carcinomas of the series and 20 otherwise conventional carcinomas, we selected neoplastic cells by manual microdissection from the corresponding paraffin blocks. A PCR-ELISA technique for human herpesvirus family detection was performed (Herplex™ Genomica, SAU, Madrid, Spain) according to manufacturer's instructions.20 Briefly, we extracted DNA from the microdissected 5-μm sections from paraffin blocks, and a PCR-ELISA procedure was performed. The PCR mix contained primers against a 194 bp sequence of DNA viral polymerase from herpes simplex types 1 and 2, varicella zoster, cytomegalovirus, EBV and human herpesvirus 6. The nucleotides present in the mix were previously labelled with digoxigenin. The PCR product was transferred to a microtiter plate where the probes against the corresponding virus were fixed previously. An antidigoxigenin antibody was added after several washes. A microtiter reader with a 405 nm light source was used to measure absorbance. Absorbance values higher than 0.450 were considered as positive. Negative and positive controls were used in every case to discharge the contamination feasibility and the specificity of the PCR reaction (data not shown). An internal control (DNA human polymerase gene) was included to assure the integrity of the sample and the amplification process.
In Situ Hybridization for EBER1 and EBER2
We used a chromogenic in situ hybridization technique (EBER Histosonda, VitroSP, Madrid, Spain) according to manufacturer's instructions. Briefly, after deparaffination, xialinized slides were immersed in H2O2 to inhibit endogenous peroxidase activity. A 30 μg/ml proteinase K digestion for 10 min at room temperature was performed. One-hour incubation with EBER probe at 62°C was performed in a humid chamber. This kind of probe consists of a fragment of single stranded 526 base long DNA marked with digoxigenin complementary to EBER1 and EBER2. Posthybridization washes with PBS, antidigoxigenin antibody and a secondary antibody at room temperature with diaminobenzidine and Harris hematoxilin counterstaining were all applied. Negative and positive controls were used in every case to discharge the possibility of non-specific staining (data not shown).
Immunohistochemistry
Immunohistochemical staining for LMP1 (Monoclonal; dilution 1:500; Dako, Glostrup, Denmark) was performed using formalin-fixed and paraffin-embedded tissue sections with heat-induced epitope retrieval. Antigen retrieval was done by boiling sections for 90 s in 10 mM sodium citrate buffer, pH=6.0, using a pressure cooker. The EnVision+ method (Dako) was used in a Techmate 550 automated immunostainer (Biotek, Santa Barbara, CA, USA). Diaminobenzidine (Dako) was used as a chromogen. The slides were counterstained with Mayer's hematoxylin (Merck, Darmstadt, Germany) and dehydrated.
Results
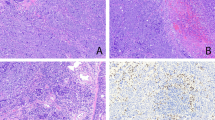
We examined 1545 pulmonary carcinomas and found 19 cases that showed a prominent inflammatory lymphoid stroma giving the neoplasm a low-power appearance of a lymphocytic proliferation (Figure 1). The limits between carcinoma cells and tumoral interspersed stroma were imprecise. Characteristically the low-power appearance was confusing between an epithelial and a lymphoid neoplasm with lymphocytes infiltrating solid carcinomatous islands giving a similar image to lymphoepithelial complexes. The clinicopathologic properties of the 19 patients are presented in Table 2. The mean age of presentation was 64.6 with a median of 67. All but one patient were males. Of the 19 cases, 13 (68%) were diagnosed in early clinicopathologic stages (UICC I and II). We could only find serology results in two cases, one adenocarcinoma and one lymphoepithelioma like that were both IgG-positive.
(a) Left lung from a case of squamous-cell carcinoma with diffuse lymphoid stroma (arrow). (b) Lymphoepithelioma-like lung carcinoma. Indistinct limits between neoplastic groups (arrows) and tumoral interspersed inflammatory stroma (H&E, original magnification × 25). (c) Squamous-cell carcinoma with strong inflammatory response. Note squamous pearl formation (arrow; H&E, original magnification × 25). (d) Adenocarcinoma with diffuse lymphoid stroma. Note the tubuloglandular pattern (H&E, original magnification × 25).
Five cases showed a typical syncytial growth pattern of undifferentiated neoplastic epithelial cells, with vesicular nuclei and prominent nucleoli without any tubuloglandular or squamous differentiation (Figure 1b). Six carcinomas demonstrated at least focally intercellular bridges and keratinization (Figure 1c) and the other eight displayed a tubuloglandular pattern (Figure 1d) with mucin production.
Ten cases (four squamous-cell carcinomas and six adenocarcinomas) showed EBV DNA sequences in the PCR-ELISA analysis (Figure 2). The same 10 carcinomas were also EBER1 and EBER2 positive by ISH analysis (Figure 3). Only 3 out of these 10 ISH-positive neoplasms were immunoreactive for LMP1 in scattered cells (Figure 4). All the PCR and/or EBER negative cases were also LMP1 negative. The EBER ISH analysis was also positive in scattered lymphoid cells intermingled with neoplastic foci.
(a and b) Squamous-cell carcinoma associated with diffuse lymphoid stroma and EBV. Note the brown nuclei in squamous-cell nests (EBER1-2 in situ hybridization. Original magnification × 100). (c and d) Adenocarcinoma with diffuse lymphoid stroma EBV associated. Brown-stained nuclei in most of neoplastic cells (EBER1-2 in situ hybridization. Original magnification × 100).
Two adenocarcinomas that gave negative results in PCR-ELISA analysis displayed a discrete reactivity for EBER. These two cases did not reacted with LMP1. Thus, total number of carcinomas that showed at least one positive technique for EBV detection were 12, and all of them belonged to adenocarcinoma or squamous-cell carcinoma categories. None of the lymphoepithelioma-like carcinomas demonstrated positivity for any of the EBV detection methods.
There were no differences neither in epidemiologic nor in staging data between PCR-positive and -negative cases. (Table 2).
Otherwise, none of the 70 squamous-cell carcinomas and adenocarcinomas that we analysed by ISH and immunohistochemistry gave positive results. The PCR of the 20 selected cases from surgical files were also negative.
It is also noteworthy that in multiplex molecular PCR analysis two adenocarcinomas also showed positivity for human herpesvirus 6. None of the rest of the herpesviruses analysed were positive (data not shown).
One adenocarcinoma displayed intratumoral amyloid deposition with positive Congo red staining as well as immunohistochemical reactivity for P substance. AA amyloid as well as prealbumin and microglobulin were negative in immunohistochemical analysis.
The rest of lung parenchyma analysed showed a diffuse lymphoid hyperplasia in two cases, atypical adenomatous hyperplasia in other and one case of silica-associated pneumoconiosis. In three patients a smoker's respiratory bronchiolitis pattern was evident.
Discussion
EBV is a very common human DNA virus; in fact, more than 90% of individuals worldwide have been infected by the time they reach adulthood.21 EBV is considered as a member of the gamma-herpesvirus subfamily, because of its tropism for lymphoid cells, although it can also infect epithelial cells of the oropharynx, as well as other kind of cells.22
EBV was the first human virus to be accepted as tumour associated since in 1964, Epstein and Barr described virus particles of the herpesvirus family in lymphoblastoid cells from patients with Burkitt's lymphoma.3 Several other neoplasms have also been associated with EBV as well as Hodgkin's disease, B-cell lymphomas in immunosuppressed individuals and a variety of undifferentiated carcinomas (the so-called lymphoepitheliomas). In the case of nasopharyngeal lymphoepitheliomas, it has been directly implicated in their pathogenesis, because EBV DNA is found in the epithelial cells of nearly all cases, EBV is transcriptionally active, and the viral episomes have demonstrated to be clonal in Southern blot analysis.23
Despite this body of evidence, the pathogenetic role of EBV in nasopharyngeal carcinomas is difficult to understand because most of otherwise healthy individuals have been infected by EBV, although the risk of developing such type of neoplasm is very low. That is the reason that some other reports have speculated about EBV presence as coincidental, due to the high prevalence of EBV infections in the general population.10 However, it must be stressed that virus particles should be detected mostly in passenger lymphoid cells in normal population and not in epithelial cells.
A variety of lymphoepithelioma-like carcinomas have been described in several other organs, with the same characteristics than the nasopharyngeal counterpart. Primary lymphoepithelioma-like carcinoma of the lung was described in 198714 as an EBV-associated neoplasm, involving 0.9% of pulmonary cancer cases.7 The last WHO classification of lung neoplasms defined lymphoepithelioma-like carcinoma as a large-cell carcinoma variant that shows a typical syncytial growth pattern of undifferentiated neoplastic epithelial cells, with vesicular nuclei and prominent nucleoli.5 In the WHO text it is stressed that to correctly diagnose this kind of carcinoma, squamous or glandular differentiation is admitted only at the ultraestructural level. The mean age of lymphoepithelioma-like patients was 10 years less than the rest of pulmonary carcinoma patients,11 they are present mainly in non-smokers and have a controversial better survival rate.7 Most of them are located in a peripheral situation.6
All pulmonary lymphoepithelioma-like carcinomas reported in the literature showed a significant CD8 T-lymphocyte reaction corresponding with cytotoxic T-lymphocytes in the resting state.24 In our series we describe five lymphoepithelioma-like cases with a similar lymphoid infiltrate that displayed characteristic vesicular nuclei. The adenocarcinoma and squamous-cell carcinomas nuclear features, however, were different with a coarse and irregular chromatin pattern. Moreover, a tubuloglandular pattern or intercellular bridges with focal squamous-cell differentiation were evident. The feature that made the difference with conventional carcinomas was the prominent lymphoid infiltration, the confusing initial appearance, because tumoral cells were difficult to see at low-power magnification, and the marked lymphoid infiltration of neoplastic islands mimicking lymphoepithelial complexes.
The relationship between lymphoepithelioma-like lung carcinomas and EBV is not clear at the moment; in fact, free EBV DNA can be detected in the serum of Oriental patients with lymphoepithelioma-like lung carcinoma, and can be used for monitoring response to therapy in advanced cases.13, 12 However, the neoplastic cells had an extremely low7 LMP1 expression, and in situ hybridization for EBV early RNA (EBER) and clonal episomal EBV sequences have been demonstrated in tumour tissue, but only in Asian9, 23, 12, 25, 26 and in two caucasic patients.18, 19 We agree with these data because our five cases of pure lymphoepithelioma-like carcinomas have been negative for EBV.
To date the association of EBV with lung carcinomas, not classified as lymphoepithelioma like, is restricted to six cases of squamous-cell carcinomas in Chinese patients,11 three squamous-cell carcinomas and two adenocarcinomas in Japanese patients,17 and the largest series in a Chinese population with 36 EBER-positive cases.16 Only two pulmonary-carcinoma EBV associated in Caucasian patients have been recently reported.18, 19 Otherwise, several cases of lymphocyte rich adenocarcinomas but without EBV association have been also reported.27, 15
We describe 12 lung carcinomas (4 squamous-cell carcinomas and 8 adenocarcinomas) in an occidental population, all of them with EBV sequences in tumoral tissue demonstrated by PCR and/or EBER. We do not exactly know the role of EBV in these kinds of carcinomas. It could be argued that EBV might only be a passive passenger. This fact could be possible if we had not demonstrated that the virus is present in neoplastic cells with EBER. In fact, there is one report where scattered EBER-positive bystander lymphocytes were found in lung carcinomas,28 as we have also shown. Moreover, we stressed the fact that all cases were selected from 1545 pulmonary carcinomas according to specific morphological characteristics, distinguished in H&E-stained sections. In consequence, we have applied morphological criteria to select a series of adenocarcinomas and squamous-cell carcinomas that have confirmed distinctive molecular properties such as the presence of EBV sequences in neoplastic tissue. Once again, optical microscopy reflects molecular pathology findings.
The most reliable method reported in the literature for detecting latent EBV in this kind of carcinomas has been in situ hybridization for EBER.7 EBER is a short non-polyadenilated chain of RNA that does not translate to protein consisting in two fragments known as EBER1 and EBER2. Their expression is nuclear and although its function is unknown it is strongly believed that they have an oncogenic role.29 Our PCR-based analysis has shown to be concordant with the EBER results. The semiquantitative results also differentiate between passenger and productive viral presence as absorbance values indicate DNA-relative quantity. The low LMP1 reactivity in these kinds of carcinomas is compatible with other reports indicating an absence or low expression rate of this protein.11
And lastly, we have also described one interesting finding: intratumoral amyloid deposition in one case. This phenomenon has been reported in 12% of nasopharyngeal carcinomas30 and in two pulmonary lymphoepithelioma-like carcinomas.7
Thus, we believe that the morphological and molecular features of EBV-associated lung carcinomas are not restricted to the typical lymphoepithelioma-like type.17 It seems that EBV infection may play a role in the tumorigenesis of some lung carcinomas. Another important question to be addressed is the difference between Oriental and Caucasian patients in EBV susceptibility for this kind of neoplasia development.
In summary, we have presented five cases of otherwise typical lymphoepithelioma-like lung carcinomas with the characteristic morphological and molecular features. They must be differentiated from squamous-cell carcinoma and adenocarcinoma variants, with a prominent lymphoid infiltrate and EBV association.
Conflict of interest
None of the authors has conflicts of interest to disclose.
References
Pershagen G, Akerblom G, Axelson O, et al. Residential radon exposure and lung cancer in Sweden. N Eng J Med 1994;330:159–164.
Veglia F, Vineis P, Overvad K, et al. Occupational exposures, environmental tobacco smoke and lung cancer. Epidemiology 2007;18:769–775.
Epstein MA, Achong BG, Barr YM . Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet 1964;1:252.
Iezzoni JC, Gaffey MJ, Weiss LM, et al. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol 1995;103:308–315.
Travis WD, Brambilla E, Müller-Hermelink HK, et al. WHO Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC: Lyon, 2004.
Chan JK, Hui PK, Tsang WY, et al. Primary lymphepithelioma-like carcinoma of the lung. A clinicopathologic study of 11 cases. Cancer 1995;76:413–422.
Chang YL, Wu CT, Shih JY, et al. New aspects in clinicopathologic and oncogene studies of 23 pulmonary lymphoepithelioma-like carcinomas. Am J Surg Pathol 2002;26:715–723.
Han AJ, Xiong M, Zong YS . Association of Epstein-Barr virus with lymphoepithelioma-like carcinoma of the lung in southern China. Am J Clin Pathol 2000;114:220–226.
Castro CY, Ostrowski ML, Barrios R, et al. Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopatologic study of 6 cases and review of the literature. Hum Pathol 2001;32:863–872.
Wockel W, Hofler G, Popper HH, et al. Lymphoepithelioma-like carcinoma of the lung. Pathol Res Pract 1995;191:1170–1174.
Chen FF, Yan JJ, Lai WW, et al. Epstein-Barr associated nonsmall cell lung carcinoma: undifferentiated lymphoepithelioma-like carcinoma as a distinct entity with better prognosis. Cancer 1998;82:2334–2342.
Conway EJ, Hudnall SD, Lazarides A, et al. Absence of evidence for an etiologic role for Epstein-Barr in neoplasms of the lung and pleura. Mod Pathol 1996;9:491–495.
Ngan RK, Yip TT, Cheng WM, et al. Circulating Epstein-Barr virus DNA in serum of patients with lymphoepithelioma-like of the lung: a potential surrogate marker for monitoring disease. Clin Cancer Res 2002;8:986–994.
Begin LR, Eskandari J, Joncas J, et al. Epstein-Barr virus related lymphoepithelioma-like carcinoma of the lung. J Surg Oncol 1987;36:280–283.
Tsuta K, Ishii G, Kim E, et al. Primary lung adenocarcinoma with massive lymphocyte infiltration. Am J Clin Pathol 2005;123:547–552.
Li CN, Han GL, Zhang SJ . Detection of Epstein-Barr virus in lung carcinoma tissue by in situ hybridization. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2007;21:288–290.
Kasai K, Kon S, Sato N, et al. Case report of lymphoepithelioma-like carcinoma of the lung-lympoid population consisting of cytotoxic T cells in resting state. Pathol Res Pract 1999;195:773–779.
Huber M, Pavlova B, Muhlberger H, et al. Detection of the Epstein-Barr virus in primary adenocarcinoma of the lung with signet ring cells. Virchows Arch 2002;441:25–30.
Morbini P, Riboni R, Tomaselli S, et al. EBER and LMP1 expressing pulmonary lymphoepithelioma-like carcinoma in a Caucasian patient. Hum Pathol 2003;34:623–625.
Brainard JA, Greenson JK, Vesy CJ, et al. Detection of cytomegalovirus in liver transplant biopsies. A comparison of light microscopy, immunohistochemistry, duplex PCR and nested PCR. Transplantation 1994;57:1753–1757.
Mendelsohn J, Howley PM, Israel MA, et al. The Molecular Basis of Cancer. 2nd edn. Saunders: NY, 2002.
Sánchez-Velasco P, Ocejo-Vinyals JG, Flores R, et al. Simultaneous multiorgan presence of human herpesvirus 8 and restricted lymphotropism of Epstein-Barr virus DNA sequences in a human immunodeficiency virus-negative immunodeficient infant. J Infect Dis 2001;183:338–342.
Pittaluga S, Wong MP, Chung LP, et al. Clonal Epstein-Barr virus in lymphoepithelioma-like carcinoma of the lung. Am J Surg Pathol 1993;17:678–682.
Kasai K, Sato Y, Kameya T, et al. Incidence of latent infection of Epstein-Barr virus in lung cancers. An analysis of EBER1 expression in lung cancers by in situ hybridisation. J Pathol 1994;174:257–265.
Wong MP, Chung LP, Yuen ST, et al. In situ detection of Epstein-Barr virus in non-small cell lung carcinomas. J Pathol 1995;177:233–240.
Butler AE, Colby TV, Weiss L, et al. Lymphoepithelioma-like carcinoma of the lung. Am J Surg Pathol 1989;13:632–639.
Minami Y, Iijima T, Onizuka M, et al. Pulmonary adenocarcinoma with massive lymphocyte infiltration: report of three cases. Lung Cancer 2003;42:63–68.
Brouchet L, Valmary S, Dahan M, et al. Detection of oncogenic virus genomes and gene products in lung carcinoma. Br J Cancer 2005;92:743–746.
Komano JS, Maruo K, Kurozumi T, et al. Oncogenic role of Epstein-Barr virus encoded RNAs in Burkitt's lymphoma cell line Akata. J Virol 1999;73:9827–9831.
Prathrap K, Looi LM, Prasad U . Localized amyloidosis in nasopharyngeal carcinoma. Histopathology 1984;8:27–34.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Román, J., Martínez, M., Fernández, S. et al. Epstein–Barr virus-associated adenocarcinomas and squamous-cell lung carcinomas. Mod Pathol 22, 530–537 (2009). https://doi.org/10.1038/modpathol.2009.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.7
Keywords
This article is cited by
-
Identification and Characterization of Epstein-Barr Virus Genomes in Lung Carcinoma Biopsy Samples by Next-Generation Sequencing Technology
Scientific Reports (2016)
-
Pulmonary adenocarcinoma with massive lymphocytic infiltration: a case report with review of the literature of a rare histological entity with a peculiar biological behaviour
BMC Pulmonary Medicine (2013)