Abstract
Background: Adenosquamous carcinoma is a rare aggressive subtype of pancreatic adenocarcinoma. We describe the clinical, pathologic, and molecular characteristics of 25 of these lesions, the largest series to date. Methods: Twenty-five cases of adenosquamous carcinoma of the pancreas diagnosed between 1961 and 1994 were retrieved from the files of the Endocrine Registry of the Armed Forces Institute of Pathology. Histologic features were reviewed, histochemical, immunohistochemical, and molecular (k-ras) studies were performed, and patient follow-up was obtained. Results: The patients included 17 men and eight women, aged 28 to 82 years (mean, 65.4 y). The patients usually experienced weight loss (n = 17) or painless jaundice (n = 11), while also presenting with other abdominal symptoms. The tumors affected the head most frequently (n = 17), followed by the tail (n = 9) or body (n = 4). Five cases involved more than one anatomic region of the pancreas. Microscopically, all tumors demonstrated dual differentiation toward adenocarcinoma and squamous cell carcinoma. All cases tested were immunoreactive with keratin (AE1:AE3 and CK1), whereas other keratin markers were variably expressed: CK5/6 (88%), CK7 (68%), Cam5.2 (41%), and CK20(26%). CA-19–9 (84%) and CEA (74%) were positive in the majority of the cases. K-ras oncogene mutations were identified in seven of 13 cases. All patients died from their disease an average of 5.8 months after diagnosis (range, 1 to 33 months). Conclusions: Adenosquamous carcinoma of the pancreas represents a distinct clinical and pathologic entity, demonstrating the expected immunoprofile and k-ras oncogene mutation of a ductal origin, with a worse prognosis than ductal adenocarcinoma.
Similar content being viewed by others
INTRODUCTION
Carcinoma of the pancreas has an incidence of 7.5 to 10 per 100,000 person years and is one of the more lethal malignancies in humans, accounting for the fourth highest cause of cancer deaths in the United States (1). Adenosquamous carcinoma, which has been called adenoacanthoma (2, 3) and mucoepidermoid carcinoma (4), is a rare subtype, accounting for 1 to 4% of exocrine malignancies (5, 6, 7), and is characterized by the histologic patterns of both ductal adenocarcinoma and squamous carcinoma within the same tumor. Despite isolated case reports or small series published on this tumor type (3, 5, 8, 9) and statistical inclusion in general studies of pancreatic exocrine neoplasms (2, 6), there is no large clinicopathologic series to date, and no series that specifically addresses the histochemical, immunohistochemical, and molecular profile of these uncommon tumors. Furthermore, there is no correlation of these results with the clinical presentation and patient outcome. We describe a series of 25 patients with adenosquamous carcinoma to specify the clinical findings associated with these tumors, illustrate their pathologic features, document their immunophenotype, present their K-ras oncogene profile, and analyze these data as they relate to patient outcome in a single comprehensive study. Our results are analyzed in comparison with a review of the English literature (MEDLINE 1966–2000).
MATERIALS AND METHODS
Twenty-five cases of adenosquamous carcinoma of the pancreas were selected from the files of the Endocrine Tumor Registry of the Armed Forces Institute of Pathology, Washington, DC, between 1961 and 1994. The cases were recovered from a search of 8,372 (0.3%) benign and malignant primary pancreatic neoplasms seen in consultation during this time. Twenty-four of the specimens were from hospitals within the United States, and one was from a military base in Europe. Ten cases were obtained from military hospitals, 10 from Veterans Administration medical centers, and five from civilian sources including four community hospitals and one university hospital. Four additional cases were identified by the search, but were excluded because of a significant component of anaplastic carcinoma within the primary lesion.
Adequate follow-up was a prerequisite for inclusion in the study. Materials within the AFIP files were supplemented by a review of the patient demographics, symptoms at presentation, medical history including radiation exposure, surgical pathology and operative reports, cancer registry records, and by written questionnaires or oral communication with the treating physician(s). Follow-up data included the exact location of the primary, the pattern of metastatic spread, the specific treatment modalities used, and the length of survival. We were unable to obtain accurate staging information on the cases due to the referral nature of our practice. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of the Code of Federal Regulations, Title 45, Part 46, and the Department of Defense Directive 3216.2 relating to human subjects in research.
Hematoxylin and eosin-stained slides from all cases were reviewed to confirm that histopathologic criteria for the diagnosis of adenosquamous carcinoma were met. Any degree of definitive squamous differentiation within an adenocarcinoma was considered sufficient for the diagnosis of adenosquamous carcinoma. Squamous differentiation was defined as solid nests or sheets of cells with abundant cytoplasm, distinct cellular borders, and intercellular bridges, with or without extracellular keratin production. Mucicarmine stains were used to confirm mucin production by the adenocarcinoma fraction of the tumors. Fifteen of the consults were autopsies, and 10 were surgical specimens, either resections or biopsies.
Immunophenotypic analysis was performed in 19 cases with suitable material. The standardized avidin-biotin method of Hsu et al. (10) was employed, using 4-μm thick, formalin-fixed, paraffin-embedded sections taken from a single representative block. In five cases immunophenotypic expression was determined on the primary tumor as well as the metastatic deposit. Table 1 documents the panel of commercially available immunohistochemical antibodies used. When required, proteolytic antigen retrieval was performed by predigestion for 3 minutes with 0.05% protease VIII (Sigma Chemical Co., St. Louis, MO) in a 0.1 m phosphate buffer at a pH of 7.8 at 37°C. Antigen enhancement (recovery) was performed, as required, using formalin-fixed, paraffin-embedded tissue treated with a buffered citric acid solution and heated for 20 minutes in a calibrated microwave oven (11). After this, the sections were allowed to cool at room temperature in a citric acid buffer solution for 45 minutes before the procedure was continued. Standard positive controls were used throughout, with serum used as the negative control. A positive reaction was determined by moderate to heavy chromogen deposition within the cytoplasm of the tumor cells.
Point mutations in K-ras-2 were sought in 15 cases. In five of the cases, metastatic lesions were analyzed as well as the primary carcinomas. Mutational analysis was performed by topographic microdissection as previously described (12, 13, 14). This type of sampling specifically selects areas of tumor from 5-μm thick paraffin-embedded tissue sections, which will yield the highest concentration of tumor cells and the lowest number of “background” normal cells. No differentiation between the glandular and squamous components was made in the sampling of the tumors for molecular analysis, as the frequent mingling of the tumor elements precluded this type of separation. PCR amplification for the K-ras-2 exon 1 gene was performed using flanking intron primers (12, 13, 14). Cycle sequencing was performed using the BigDye terminator cycle sequencing approach (Perkin-Elmer, Foster City, CA) with one of the amplifying primers, and subsequently run on an ABI Prism 377 automated sequencer (Perkin-Elmer) using a 6% denaturing polyacrylamide gel. Suspect mutations were subsequently re-amplified and confirmed by sequencing the opposite strand.
A review of English journal publications (1966 to 2000) was performed, and all cases of adenosquamous carcinoma (adenoacanthoma and mucoepidermoid carcinoma were also reviewed) were included in the review, even if part of a large review series. However, our summary of the literature is confined to reports that provide sufficient clinical and pathology description to confirm the diagnosis. No foreign language articles were included.
RESULTS
Clinical
The demographic data of the patients are summarized in Table 2. Eighteen patients reported weight loss, 13 presented with jaundice, and 13 complained of abdominal symptoms, which included pain, nausea, vomiting, bleeding, bloating, and a palpable mass. The duration of symptoms ranged from 1 to 27 months, with a mean duration of 5.4 months. The patients with the longest duration of symptoms had nonspecific symptoms of bloating and nausea.
Pathology
Macroscopic
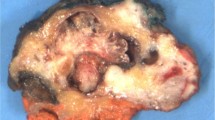
The tumors occurred in the head of the pancreas (n = 17), the tail (n = 9), and the body (n = 4). Among these, five tumors encompassed multiple anatomic segments of the pancreas, with one case involving the entire pancreas. The pancreatic tumors ranged in size from 2 to 12 cm in greatest dimension, with a mean size of 6.3 cm. Information about the size of the primary was unavailable for four of the cases. Macroscopically, the tumors were described as infiltrative yellow-white, gray, or green firm masses with areas of softening or liquefaction. Three of the masses were multinodular and two were cystic. The cut surface was described as fibrous or gritty, and a minor number extruded mucinous material.
Microscopic
All tumors exhibited a biphasic malignant growth identified as well to poorly differentiated adenocarcinoma and well to poorly differentiated squamous cell carcinoma. The adenocarcinoma component contained ductal or glandular structures with focal to abundant intracellular or extracellular mucin (Fig. 1A–B). Rare tumors contained foci of clear cell adenocarcinoma or signet cell carcinoma. Squamous differentiation was characterized by irregular and infiltrating nests or sheets of polygonal cells with distinct cellular borders, intercellular bridges, opaque eosinophilic cytoplasm, and varying degrees of keratinization (Fig. 1C–D). These two different patterns could be seen separated topographically within the substance of the tumor or intimately amalgamated with one another (Fig. 2). Benign squamous metaplasia in the surrounding pancreatic tissue was absent in all of the cases. No “intermediate” cells like the type seen in mucoepidermoid carcinoma of the salivary gland were evident in any of the cases. Perineural or neural invasion was identified in 20 of the tumors (Fig. 3) and both large vessel and lymphatic invasion were common.
The morphologic spectrum of glandular and squamous components. Adenocarcinoma similar to conventional ductal carcinoma (A), but rare cases were clear cell (B). Squamous cell carcinoma composed of irregular nests of polygonal cells with no gland formation. A well-differentiated SCC with abundant keratin (C). Keratin was absent in a moderate to poorly differentiated tumor (D).
The two types of carcinoma were present in variable proportions, with the minority of tumors exhibiting almost exclusive squamous cell phenotype. However, adenocarcinoma was always present somewhere within the tumor, and was confirmed in all cases by a positive mucicarmine stain (Fig. 4). We reviewed the metastatic deposits in 13 cases (pathology slides or blocks of the metastatic foci were not available in the remaining cases), nine of which were exclusively adenocarcinoma. Interestingly, three of the carcinomas underwent a dedifferentiation on metastasizing and exhibited the giant cell or pleomorphic cell-type anaplastic carcinoma. These anaplastic metastatic foci were collections of loosely cohesive sheets of markedly enlarged cells with bizarre nuclei, prominent nucleoli, and multinucleated forms (Fig. 5). Squamous cell carcinoma was only identified focally (less than 5% of the volume) in the metastatic lesions of two cases.
Immunohistochemistry
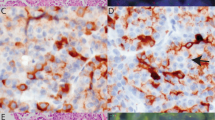
Tissue blocks were available in 19 cases. In five of the cases, tissue from metastatic lesions was available, and immunohistochemistry was performed on the metastatic lesions as well as the primary lesions. All cases were positive for the pan-cytokeratin (Table 3). The majority of the tumors (68%) expressed CK7, whereas only a few cases (16%) yielded focal reactivity to anti-CK20. All cases that were immunoreactive with anti-CK20 were more diffusely and intensely reactive for CK7. Immunoreactivity for CK7 and CK20 was restricted to the adenocarcinoma component. CK5/6 was reactive (88% of cases tested) predominantly in the squamous cell component (Fig. 6). Cam 5.2 showed a slightly greater predilection for adenocarcinoma and was positive in 47% of the cases. Whereas CA 19–9 stained both components (89% of cases tested), the adenocarcinoma reacted with a greater intensity and distribution than the squamous cell carcinoma, which tended to react in the central areas of the squamous nests (Fig. 7). Likewise, CEA had an almost identical pattern of distribution as CA19–9, while reactive in 79% of cases. All cases were negative for chromogranin.
Molecular Pathology
K-ras oncogene mutational analysis was performed on 13 cases with amplifiable DNA. Six cases showed a heterozygous mutation at codon 12, of which two cases had metastases: one had a heterozygous mutation identified in both the primary and metastasis, and the other had a heterozygous mutation in the primary but not in the metastasis. One additional case contained a homozygous mutation in the metastatic focus alone. The mutations caused a change in the amino acid product of codon 12 from glycine to valine in three of the cases and from glycine to aspartate in the other four, including the homozygous mutation. No K-ras oncogene mutation was present in six cases (wild type), including two cases with metastatic lesions. The different morphologies of the tumors are compared with the oncogene mutations in Table 4. There was no appreciable correlation between the presence of K-ras mutations and the proportion of squamous and adenocarcinomatous elements.
Treatment and Follow-Up
Twelve patients received no surgical therapy. Five patients received palliative bypass surgery to alleviate symptoms of bile duct obstruction, whereas eight patients had a partial or total pancreatectomy. Four patients had an initial biopsy of the pancreatic mass, followed by bypass surgery (n = 2), resection (n = 1), or no additional procedure (n = 1). Only four patients accepted chemotherapy: two after partial pancreatectomy, one after a choledochojejunostomy bypass, and one who underwent no other treatment (Table 5). The overall survival was 12.5 months for patients treated with chemotherapy and 4.5 months for the patients who received none. Radiation therapy was not used for any patients.
The presence of K-ras oncogene mutations was correlated with survival, as presented in Table 4. The seven patients whose tumors had codon 12 mutations survived for an average of 8 months and the patients whose tumors expressed a wild-type phenotype survived for an average of 7 months.
All of the patients died as a result of their disease, irrespective of the type of therapy instituted. Survival after diagnosis ranged from 1 to 33 months, with a mean of 6 months. Only three patients survived beyond 1 year, all of whom had submitted to a Whipple procedure. Widely disseminated metastatic disease was the most common cause of death; other causes included disseminated intravascular coagulopathy, acute gastrointestinal bleeding, pulmonary embolism, pneumonia, septic shock, and end-stage liver disease. Metastatic disease was identified in the local lymph nodes (n = 13 cases), distant lymph nodes (n = 6 cases), and other solid organ sites (n = 14), including liver, lung, adrenal, brain, stomach, kidney, esophagus, and peritoneum. Direct extension by the tumor into the common bile duct, spleen, porta hepatis, and gallbladder was documented clinically or microscopically.
DISCUSSION
Adenosquamous carcinoma is included among the histologic variants of pancreatic carcinoma that have been delineated in several studies (6, 15, 16). As mentioned above, a number of synonyms have been applied to adenosquamous carcinoma since its initial description as “cancroide.” (17) Adenosquamous carcinoma is distinctive, separable from the more common ductal adenocarcinoma, as it demonstrates both malignant squamous cell and glandular differentiation.
Malignant tumors that contain both squamous cell and glandular elements are described in a number of different anatomic sites, including the respiratory tract, gastrointestinal tract, genitourinary tract, pancreaticobiliary tract, salivary glands, and thyroid (3, 5, 18). Whereas a few sites, such as the lung and esophagus, may develop either adenocarcinoma or squamous cell carcinoma as a primary tumor, most of the other locations almost exclusively give rise one type or the other. Based on the findings of this study, we subscribe to the theory that adenosquamous carcinomas in the pancreas occur as a result of malignant squamous metaplastic change of an adenocarcinoma (3).
Squamous metaplasia of the pancreatic ductal epithelium occurs most commonly in the setting of chronic pancreatitis, but is noted in the adjacent ducts of only about 4% of adenocarcinomas (15). Similar to the literature, we were unable to demonstrate either squamous metaplasia in the uninvolved pancreas or a transition from squamous metaplasia to squamous cell carcinoma. By contrast, a spectrum of ductal hyperplasia to carcinoma in situ was easily identified in cases of adenocarcinoma, both in this series and in reports in the literature (15). The propinquity and often intimate intermingling of the squamous cell and ductal elements of the cases in this series morphologically dispels the notion of a collision tumor. K-ras oncogene mutations, present in ductal adenocarcinoma, have also been used to prove the ductal histogenesis of anaplastic carcinoma (19). Therefore, by inference, the presence of K-ras oncogene mutations in over 50% of adenosquamous carcinomas we analyzed, even within tumors that were almost exclusively squamous, suggests a ductal origin for both elements of adenosquamous carcinomas. This incidence of mutation is lower than that reported in the literature for adenocarcinoma. However, the method we employed for detection does not include a restriction step to enhance recovery of K-ras mutations, and only yields a positive result when approximately 20% or more of the cells analyzed harbor a mutation. Based on preliminary work at our institute, this less sensitive detection of K-ras mutations in pancreatic adenocarcinoma may identify mutations that are more significantly correlated to tumor biology (unpublished data).
All of the mutations in this series occurred at codon 12, the principle locus of K-ras mutations in ductal adenocarcinoma. Moreover, the mutation does not correlate with tumor morphology because K-ras abnormalities were noted in tumors that were intermingled, contained a dominant squamous cell differentiation, or had a predominantly glandular differentiation. Therefore, based on these findings, the presence of K-ras oncogene mutation suggests a ductal histogenesis of adenosquamous carcinoma and does not have specific prognostic or diagnostic implications.
The exact proportion of squamous cell differentiation required to diagnose adenosquamous carcinoma is variable, although arbitrarily set by a few authors at 30% of the routinely sectioned tumor volume (5, 7). The percentage of squamous cell carcinoma within this series varied, from predominantly squamous cell carcinoma to mostly adenocarcinoma. Whereas we did not prosect the specimens and so could not meticulously map the tumor topography as others have (20), we did not find a consistent topical distribution of the two components. We believe that accurate evaluation of the percentages is too subjective to be reliably reproduced based on the amount of tumor sectioned, the specific area selected, and the method used to determine the tumor proportions. It is our opinion that primary tumors of the pancreas that show any degree of definitive malignant squamous cell differentiation on routine sectioning should be considered adenosquamous carcinoma. Furthermore, it has been suggested in the medical literature that squamous cell carcinoma occurs de novo within the pancreas without an identifiable ductal component (16, 21, 22, 23). Based on our review of these publications, there was either limited diagnostic material or no resection specimen, or the cases were presented as part of a large series of pancreatic cancer, which did not specify the details of the individual cases. In our experience, we have yet to identify a primary pure squamous cell carcinoma of the pancreas, and if such an entity exists it is vanishingly rare. With adequate tissue processing we believe a ductal component is almost always present even in tumors that show a dominant squamous cell morphology.
Perineural infiltration is a common feature of adenosquamous carcinoma, occurring in 80% of the cases in this series, which is a higher incidence than has been previously reported (21). As with conventional adenocarcinoma, this feature is helpful in diagnosing malignancy on core biopsies, but shows no prognostic significance. Although we noted both large vessel and lymphatic spread in the tumors, we did not quantify the percentage of cases with vascular invasion because of its lack of impact on the prognosis of these aggressive neoplasms and because of interobserver variability in interpretation, particularly in small biopsy specimens.
Immunophenotypically, the glandular and squamous cell areas were distinctive. Keratin expression differed, with the adenocarcinoma reacting with anti-CK7 and the squamous cell carcinoma reacting with anti-CK5/6. The CK7 immunoreactivity of the adenocarcinoma component duplicates that of normal pancreatic ducts and conventional adenocarcinoma of the pancreas (24, 25). The tumor markers CA19–9 and pCEA are thought to represent markers of adenocarcinoma, and predominantly stained the glandular component, but also demonstrated focal immunoreactivity in squamous cell areas. This differential antigen expression should not be construed as proof of separate clonality or origin, but rather as different phenotypic expression within a single neoplastic proliferation.
The distinction of adenosquamous carcinoma from ductal adenocarcinoma may have certain clinical relevance, even though the clinical presentation and epidemiology of the patients in this series are similar to conventional ductal adenocarcinoma (26). Whereas the prognosis for ductal adenocarcinoma of the pancreas is grim, with a 3 to 5% 5-year survival rate (26, 27), the overall survival for adenosquamous carcinoma appears to be worse. Only three patients in this series survived for at least 1 year after diagnosis, with an overall average survival of less than 6 months. Specifically, the 5-year survival rate of resectable adenocarcinomas is 10% with a median survival of 18 to 20 months (26), while the eight patients in this series who received complete surgical resection had an average survival of only 11 months. Although the number of cases in this series is too small to demonstrate statistical significance to this discrepancy, the data at least suggest a more rapid clinical course. The aggressive behavior of adenosquamous carcinoma is analogous to that of anaplastic carcinoma. These two entities are likely related, and represent variant degrees of differentiation of ductal adenocarcinoma. The three metastatic tumors in this series that exhibited anaplastic carcinoma, two of which demonstrating a K-ras oncogene mutation, support this hypothesis. Similarly, we rejected four additional cases from our search because they contained a large proportion of anaplastic carcinoma in the primary tumor. The overall survival of the patients is not affected by quantifiable molecular mutations. The longest surviving patient had a tumor with a heterozygous K-ras mutation, whereas many patients with carcinomas expressing a wild-type genome died rapidly from metastatic disease.
The usual aggressive clinical course could affect decisions regarding therapy. With the reliability, safety, and increasing popularity of intraoperative or transabdominal fine-needle aspiration biopsy, adenosquamous carcinoma may be diagnosed by cytology before resection (8, 28). As such, this may introduce another variable in deciding whether to undergo major abdominal surgery when there is no chance of cure and a more rapid clinical course based on current data (5, 8). In this series, the patients who survived the longest had all undergone a Whipple resection, suggesting that this palliative treatment may extend the patient's life, and may improve the quality of life by reducing chronic pain, biliary obstruction, or duodenal obstruction (26). Alternatively, the longer survival may merely reflect the earlier stage of the primary in these cases, rendering them amenable to surgical resection. The use of chemotherapy as an adjunct to surgery may also increase the duration of survival, as is suggested in this series. However, owing to the rarity of adenosquamous carcinoma, the number of cases is too small to statistically support this claim.
References
Ahlgren J . Epidemiology and risk factors in pancreatic cancer. Semin Oncol 1996; 23: 241–250.
Sommers SC, Meissner WA . Unusual carcinomas of the pancreas. Arch Pathol Lab Med 1954; 58: 101–111.
Cihak RW, Kawashima T, Steer A . Adenoacanthoma (adenosquamous carcinoma) of the pancreas. Cancer 1972; 29: 1133–1140.
Frantz WK . Adenosquamous carcinoma. In: Frantz WK, editors. Tumors of the pancreas. Atlas of tumor pathology. Series 1 Fascicle 8. Washington, DC: Armed Forces Institute of Pathology; 1959. p. 66.
Madura JA, Jarman BT, Doherty MG, Yum MN, Howard TJ . Adenosquamous carcinoma of the pancreas. Arch Surg 1999; 134: 599–603.
Cubilla AL, Fitzgerald PJ . Morphological patterns of primary nonendocrine human pancreas carcinoma. Cancer Res 1975; 35: 2234–2248.
Solcia E, Capella C, Klöppel G . Adenosquamous carcinoma. In: Solcia E, Capella C, Klöppel G, editors. Tumors of the pancreas. Atlas of tumor pathology. Series 3 Fascicle 20. Washington, DC: Armed Forces Institute of Pathology; 1995. p. 90–91.
Wilczynski SP, Valente PT, Atkinson BF . Cytodiagnosis of adenosquamous carcinoma of the pancreas. Use of intraoperative fine needle aspiration. Acta Cytol 1984; 28: 733–736.
Aranha G, Yong S, Olson M . Adenosquamous carcinoma of the pancreas. Int J Pancreatol 1999; 26: 85–91.
Hsu SM, Raine L, Fanger H . Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981; 29: 577–580.
Tacha DE, Chen T . Modified antigen retrieval procedure: calibration technique for microwave ovens. J Histotech 1994; 17: 365–366.
Przygodzki RM, Finkelstein SD, Keohavong P, Zhu D, Bakker A, Swalsky PA, et al. Sporadic and Thorotrast-induced angiosarcomas of the liver manifest frequent and multiple point mutations in K-ras-2. Lab Invest 1997; 76: 153–159.
Przygodzki RM, Koss MN, Moran CA, Langer JC, Swalsky PA, Fishback N, et al. Pleomorphic (giant and spindle cell) carcinoma is genetically distinct from adenocarcinoma and squamous cell carcinoma by K-ras-2 and p53 analysis. Am J Clin Pathol 1996; 106: 487–492.
Przygodzki RM, Finkelstein SD, Langer JC, Swalsky PA, Fishback N, Bakker A, et al. Analysis of p53, K-ras-2, and C-raf-1 in pulmonary neuroendocrine tumors. Correlation with histological subtype and clinical outcome. Am J Pathol 1996; 148: 1531–1541.
Chen J, Baithun SI, Ramsay MA . Histogenesis of pancreatic carcinomas: a study based on 248 cases. J Pathol 1985; 146: 65–76.
Baylor SM, Berg JW . Cross-classification and survival characteristics of 5,000 cases of cancer of the pancreas. J Surg Oncol 1973; 5: 335–358.
Herxheimer G . Über heterologe Cancroide. Bietr Pathol Anat 1907; 41: 348–412.
Wenig BM, Adair CF, Heffess CS . Primary mucoepidermoid carcinoma of the thyroid gland: a report of six cases and a review of the literature of a follicular epithelial derived tumor. Hum Pathol 1995; 26: 1099–1108.
Hoorens A, Prenzel K, Lemoine NR, Klöppel G . Undifferentiated carcinoma of the pancreas: analysis of intermediate filament profile and Ki-ras mutations provides evidence of a ductal origin. J Pathol 1998; 185: 53–60.
Yamaguchi K, Enjoji M . Adenosquamous carcinoma of the pancreas: a clinicopathologic study. J Surg Oncol 1991; 47: 109–116.
Matsuya S, Pour PM . Adenosquamous carcinoma. In: Pour PM, Konishi Y, Klöppel G, Longnecker DS, editors. Atlas of exocrine pancreatic tumors. Tokyo: Springer-Verlag; 1994. p. 159–168.
Sears HF, Kim Y, Strawitz J . Squamous cell carcinoma of the pancreas. J Surg Oncol 1980; 14: 261–265.
Brayko CM, Doll DC . Squamous cell carcinoma of the pancreas associated with hypercalcemia. Gastroenterology 1982; 83: 1297–1299.
Osborn M, van Lessen G, Weber K, Klöppel G, Altmannsberger M . Differential diagnosis of gastrointestinal carcinomas by using monoclonal antibodies specific for individual keratin polypeptides. Lab Invest 1986; 55: 497–504.
Tot T . Adenocarcinomas metastatic to the liver: the value of cytokeratins 20 and 7 in the search for unknown primary tumors. Cancer 1999; 85: 171–177.
DiMagno EP, Reber HA, Tempero MA . AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. Gastroenterology 1999; 117: 1464–1484.
Tabuchi T, Itoh K, Ohshio G, Kojima N, Maetani Y, Shibata T, et al. Tumor staging of pancreatic adenocarcinoma using early- and late-phase helical CT. AJR Am J Roentgenol 1999; 173: 375–380.
Lozano MD, Panizo A, Sola IJ, Pardo-Mindan FJ . FNAC guided by computed tomography in the diagnosis of primary pancreatic adenosquamous carcinoma. A report of three cases. Acta Cytol 1998; 42: 1451–1454.
Acknowledgements
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Defense.
The authors thank Ms. Serena Lei for her editorial review of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kardon, D., Thompson, L., Przygodzki, R. et al. Adenosquamous Carcinoma Of The Pancreas: A Clinicopathologic Series Of 25 Cases. Mod Pathol 14, 443–451 (2001). https://doi.org/10.1038/modpathol.3880332
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880332
Keywords
This article is cited by
-
Spontaneously evolved progenitor niches escape Yap oncogene addiction in advanced pancreatic ductal adenocarcinomas
Nature Communications (2023)
-
Research advances and treatment perspectives of pancreatic adenosquamous carcinoma
Cellular Oncology (2023)
-
Adenosquamous carcinoma of the pancreas: two case reports and review of the literature
Journal of Medical Case Reports (2022)
-
Mucoepidermoid carcinoma (MEC) and adenosquamous carcinoma (ASC), the same or different entities?
Modern Pathology (2022)
-
Morphological and p40 immunohistochemical analysis of squamous differentiation in endoscopic ultrasound guided fine needle biopsies of pancreatic ductal adenocarcinoma
Scientific Reports (2021)