Abstract
Induction of mucosal immunity is critical for protection from enteric pathogens. Heat shock protein gp96 is one of the primary peptide and protein chaperones located in the endoplasmic reticulum. We reported previously that a cell-secreted gp96-Ig fusion protein (gp96-Ig) mediated strong systemic, antigen-specific CD8-CTL expansion in vivo. We now evaluate the mucosal immune response to stimulation by secreted gp96 using allogeneic NIH-3T3 transfected with ovalbumin (OVA) and gp96-Ig. A single intraperitoneal NIH-3T3-OVA-gp96-Ig immunization caused significant homing of OVA-specific TCR transgenic CD8 cells (OT-I) to Peyer's patches, to the intraepithelial compartment and to the lamina propria. Intraperitoneal immunization with cells secreting gp96-Ig provided stronger mucosal immunity than the same dose instilled vaginally or rectally or injected subcutaneously or intradermally. Our results provide the first evidence that cell-based gp96-Ig-secreting vaccines may serve as a potent modality to induce mucosal immunity.
Similar content being viewed by others
Introduction
Mucosal surfaces of the gastrointestinal tract directly interact with the mucosal lumen, the harshest environment in our body and one that is constantly exposed to many foreign and food antigens. Because many pathogens infect mammalian hosts through mucosal surfaces, maintenance of immunological memory within the gut provides a first line of defense against reinfection.1 Indeed, pathogen-specific CD8T cells persist within the lamina propria (LP) and intraepithelial compartment of the intestinal mucosa following the clearance of the bacterial or viral infection.2, 3, 4 The development of cellular mucosal memory may be requisite for successful vaccination against certain pathogens, such as HIV.5
An important component of adaptive immunity is the ability of effector CD4 and CD8 cells to recirculate and home to the site of infection which is usually near to, but not identical to, the site of primary activation. Mucosal effector cells are attracted by CCL25 and CCR9 and retained by α4β7 integrin/MAdCAM-1 (lamina propria lymphocyte, LPL) or αEβ7(CD103)/E-cadherin (intraepithelial lymphocyte, IEL).6 The α4β7 integrin binds to an Ig-like domain on the MAdCAM-1 addressin, which is expressed by endothelium in Peyer's patches and LP,7 whereas αEβ7(CD103) binds to E-cadherin expressed by intestinal epithelial cells.8 Mice deficient for β7 integrins have greatly reduced number of IELs.9 Mice defective for the αE subunit also have decreased IELs,10 although the decrease is less pronounced.
The molecular basis of imprinting of gut-homing lymphocytes by mucosal dendritic cells (DCs) is based on their capacity to produce the vitamin A (retinol) metabolite retinoic acid, which enhances the expression of retinoic acid-sensitive genes, including α4β7 and CCR9.11 A functionally distinct subset of CD103+ DC has recently been identified in murine and human mesenteric lymph nodes (LNs) that induce gut-homing receptor (α4β7 and CCR9) expression in responding T cells.12, 13
We have developed a vaccine design that uses the unique ability of the endoplasmic reticulum chaperone, heat shock protein gp96, also known as Grp94, to bind antigenic peptides and deliver them to antigen-presenting cells (APCs).14, 15 To generate a secreted form of gp96, we replaced the endoplasmic reticulum retention sequence KDEL of gp96-cDNA with the IgG1-Fc domain and hinge to generate the fusion protein gp96-Ig.16 Transfection of the cDNA of gp96-Ig into several cell lines (293, NIH-3T3, EG7, LLC), resulted in gp96 secretion by these cells, due to the lack of the endoplasmic reticulum retention sequence.16 Antigen specificity is provided by the peptide bound to gp96, which is taken up together with gp96 by APCs through the CD91 receptor.17 The repertoire of peptides bound by gp96 reflects the entire repertoire of peptides present inside the endoplasmic reticulum including those peptides imported by the TAP transporter.15 Only a small fraction of gp96-bound peptides, after further trimming, will be loaded onto major histocompatibility complex (MHC) class I. Peptide binding by gp96 is highly promiscuous, and the gp96 peptide repertoire is independent of the MHC molecules expressed by the cell. Therefore, isolated (cell-free) gp96 with its bound peptides, on uptake even by allogeneic DC, is ideally suited to cross-prime CD8-CTL responses against all kinds of intracellular antigens, including antigens derived from infectious agents and tumor antigens.18, 19, 20 Cell-released (cell-free) gp96 functions as potent internal adjuvant21 to signal traumatic/necrotic cell death and exerts it effect simultaneously as peptide carrier for antigen cross-presentation and cross-priming of antigen-specific CD8-CTL responses.22 Cell-free gp96 thus is a danger signal alerting DCs and other APCs through CD91,17 TLR2, TLR4,23 and SRA.24 Gp96 binding causes APC and DC activation and maturation and upregulation of B7, independent of CD40 signals: the adjuvant effect of gp96.16, 25, 26 Gp96 together with its associated peptides is engulfed through CD91 and the engulfed peptides are cross-presented by MHC class I of the engulfing APC and prime cognate CD8 cells and generate memory CD8 cells: cross-priming.17, 22, 27
In this study, we describe the role of secreted gp96-Ig in the induction of mucosal immunity. This is the first report to present evidence that gp96-Ig immunization induces antigen-specific effector memory CD8T cells migrating to the intestinal mucosa. Thus, gp96-Ig vaccination strategy could be extremely useful for improving protection against a range of mucosal pathogens.
Results
Induction of adaptive CD8αβ+TCRαβ+ antigen-specific CD8+ LPL and IEL by secreted gp96-Ig
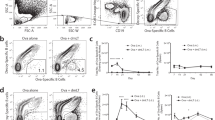
Secreted gp96-Ig peptide complexes provide within one molecular complex strong adjuvant properties to activate DC and antigen specificity for cross-presentation and cross-priming of CD8T cells.16, 22, 28 To determine whether gp96-Ig vaccination induced mucosal immunity, we used the EG7-gp96-Ig/OT-1 model developed previously.22, 28 In this model the expansion of TCR transgenic, OVA-specific OT-I CD8T cells in response to EG7-gp96-Ig immunization is quantitated at several mucosal and peripheral sites. First, we characterized the phenotype of mucosal small intestine lymphocytes from nonimmunized C57Bl/6 (B6) mice (Supplementary Figure 1). Immunized B6 mice received IV one million green fluorescent protein (gfp)-marked, TCR transgenic OT-I cells recognizing SIINFEKL (ova peptide) presented by Kb. After 2 days, mice were immunized IP with four million EG7-gp96-Ig or four million EG7 (derived from the EL4 lymphoma by OVA transfection). Four million EG7-gp96-Ig cells secrete 250 ng gp96-Ig within 24 h in culture. We found that gp96-Ig secretion mediated robust CD8-CTL (OT-I) expansion reaching a peak on day 5; expansion is detectable locally in the peritoneal cavity (PEC) and systemically in draining LNs, blood, and spleen (SPL).28 In addition to the analysis of gfp-OT-I cells in the PEC and in the SPL, the frequency of gfp-OT-I was examined within Peyer's patch lymphocytes (PPLs), IELs, and LPL. EG7 cells (secreting OVA but not gp96) had virtually no effect on OT-I homing to mucosal sites, which remained close to zero frequency (Figure 1a and b). In contrast, a single IP immunization with four million EG7-gp96-Ig cells secreting 250 ng gp96-Ig in 24 h (in culture) caused considerable homing of OT-I to Peyer's patches (2.9±1.1% s.e.m. OT-I in the CD8 gate), to the intraepithelial compartment (4.1±1% s.e.m.) and to the LP (14.9±5% s.e.m.) (Figure 1a and b). Clearly, gp96-Ig IP vaccination is a powerful inducer of antigen-specific CD8 cells at mucosal sites.
Gfp-OT-1 cells locate to the mucosa after gp96-Ig immunization. Mice received one million gfp-OT-I IV. After 2 days they received four million EG7-gp96-Ig (left) or EG7 (right panels) IP as vaccine. After 5 days, the frequency of gfp-OT-I was analyzed in Peyer's patch lymphocytes (PPL), intraepithelial lymphocytes (IEL), and lamina propria lymphocytes (LPL). (a) Representative dot plots of PPL, IEL, and LPL on day 5 after staining for CD8 and analyzing the lymphocyte gate. Numbers in the quadrants represent percent of CD8+ or CD8+gfp+OT-I cells within the lymphocyte gate. (b) OT-I expansion expressed as %±s.e.m. of CD8 cells at various sites. The number of mice per group was n=3–6. *P<0.05, **P<0.01, ***P<0.001 (compared with EG7 immunization).
Since OT-I cells are Kb restricted, direct antigen presentation to OT-I by syngeneic EG7 cells is possible. To exclude direct antigen presentation, we used allogeneic NIH-3T3 cells transfected with OVA and gp96-Ig (3T3-OVA-gp96-Ig), which can cause OT-I expansion only by MHC-class-I-mediated cross-presentation of OVA-derived SIINFEKL (Figure 2). NIH-3T3-OVA cells, not transfected with gp96-Ig, were used as controls. IP vaccination with NIH-3T3-OVA-gp96-Ig induced OT-I homing to the mucosal compartments at a similar frequency as syngeneic EG7-gp96-Ig vaccination, namely 2.7±0.8, 4.1±0.8, and 9.8±3.1% s.e.m. in PPL, IEL, and LPL, respectively (Figures 1b and 2b). Very high frequencies of OT-1 CTL were also found at the site of the injection, the PEC (up to 60% of CD8 cells, ∼400,000 total OT-I), in the SPL (5%) and mesenteric LNs (1%). It is noteworthy that NIH-3T3-OVA cells, not secreting gp96-Ig, attract relatively few CD8 cells to the PEC, only 1.4% of the cells in the lymphocyte gate are CD8+ as opposed to almost 20% CD8 cells when gp96-Ig is secreted. This observation indicates that secreted gp96-Ig triggers immune responses that are targeted toward cytotoxic responses. OT-I expansion was not found in the animals that were injected with NIH-3T3-OVA (Figure 2b) or NIH-3T3-gp96-Ig (not containing OVA; data not shown, but see Oizumi et al.28), indicating that the antigen must be present in cell secreting gp96-Ig.
NIH-3T3-OVA-gp96-Ig cells administered IP mediate strong systemic and mucosal OT-I expansion. Mice received one million gfp-OT-I IV. After 2 days they received two million NIH-3T3-OVA-gp96-Ig cells (upper panels) or 2 million NIH-3T3-OVA cells (lower panels) IP as vaccine. After 5 days, the frequency of gfp-OT-I was analyzed within intraepithelial lymphocytes (IEL), Peyer's patch lymphocytes (PPL), lamina propria lymphocytes (LPL), peritoneal cavity (PEC), spleen (SPL), and mesenteric lymph nodes (MLNs). (a) Representative dot plots of PEC, SPL, MLN, PPL, IEL, and LPL on day 5 after staining for CD8 and analyzing the lymphocyte gate. Numbers in the quadrants represent percent of positive cells within lymphocyte gate. (b) Summary of OT-I expansion in 3–6 experiments expressed as %±s.e.m. of CD8 cells at various sites. *P<0.05, **P<0.01, ***P<0.001 (compared with 3T3-OVA immunization).
Gp96-Ig immunization induces memory OT-I cells that express receptors for intestinal homing and cytotoxic molecules
Gfp-OT-I cells isolated from mucosal compartments following gp96-Ig immunization are (CD44high CD62Llow CCR7low; Figure 3) similar to the SPL and PEC (data not shown). We found that mucosal OT-I cells retained their CD8 complex composition and, as expected, remained TCRαβ+CD8αβ+ within all mucosal compartments (data not shown). Cell-secreted gp96-Ig-peptide complexes however induced upregulation of the gut-homing molecules αEβ7 (CD103), α4β7, and CCR9 (Figure 3) in OT-I isolated from mucosal compartments.
Mucosal phenotype of gp96-ova cross-primed OT-I at mucosal sites. Mice received one million gfp-OT-I IV. After 2 days they received two million NIH-3T3-OVA-gp96-Ig cells IP. After 5 days, the phenotype of gfp-OT-I was analyzed within Peyer's patch lymphocytes (PPL), intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs), and compared with naive gfp-OT-I (before transfer). (a) Each bar represents the mean±s.e. from 4 to 6 mice and (b) numbers represent the percentage of CCR9+ cells among gfp-OT-I cells before and after transfer. (c) The overlay histogram shows granzyme B expression in naive gfp-OT-I (dark gray histogram) and gfp-OT-I that had migrated to different mucosal compartments: PPL (dashed line), IEL (dotted line), LPL (solid line) 5 days after gp96-Ig vaccination. Isotype control is shown in light gray histogram. Cells from different compartments were stained for surface CD8 and than fixed/permeabilized and intracellular staining for granzyme B was performed.
Gp96-Ig vaccine also induced high levels of granzyme B in OT-I in all three gut-homing compartments, PPL, IEL, and LPL (Figure 3), suggesting that gp96-Ig-induced, antigen-specific, adaptive CTL in mucosal compartments could contribute to the elimination of antigen-specific target cells and may serve as protection against mucosal virus infection.
Antigen-specific memory CD8T cells that migrated to the SPL or the intestinal mucosa after gp96-Ig immunization differ in phenotype and function
There is evidence that the memory phenotype is coupled to anatomic location and that memory CD8T cells residing within the intestinal mucosa differ from their clonotypic counterparts within the SPL regarding phenotype, function, cell cycle, and cytokine receptor expression.29 Comparing the phenotype of gp96-Ig-induced SPL and IEL OT-I cells 5 days after gp96-Ig vaccination, we found CD62Llow CD44high cells in both populations. However a subpopulation of CD62Lhigh cells was present only within SPL OT-I (Figure 4). Further, in contrast to IEL OT-I, SPL OT-I remained CD103low CCR9low and α4β7low similar to naive OT-I. These data show for the first time that gp96-Ig induces expression of essential intestinal homing receptors on antigen-specific CTLs. Compared with the previously reported immunizations with high doses of whole OVA, or Ova peptide with adjuvant,12, 30 much lower doses of peptide bound to Gp96-Ig are required to induce OT-I activation and localization to the intestinal effector sites. The memory cells that accumulate in the intraepithelial compartment bear little resemblance to recirculating central or effector memory cells.29 Following gp96-Ig vaccination, only effector phenotypic OT-I cells migrated to the gut mucosa. LPL OT-I and IEL OT-I cells expressed the highest level of granzyme B with a mean fluorescent intensity (MFI) for granzyme B within IEL OT-I=6,976 and MFI LPL OT-I=7,333 compared with SPL MFI OT-I=5,576 (Figure 4).
Antigen-specific memory CD8T cells that migrate to spleen or intestinal mucosa after NIH-3T3-ova-gp96-Ig immunization differ in phenotype and function. Mice received one million gfp-OT-I IV. After 2 days they received two million NIH-3T3-OVA-gp96-Ig IP. After 5 days, the phenotype of gfp-OT-I cells in spleen (solid line) and in IEL (dashed line) was compared. To determine granzyme B expression, 5 days after NIH-3T3-OVA-gp96-Ig IP immunization, SPL, IEL and LPL were stained for surface CD8 and than fixed/permeabilized and intracellular staining for granzyme B was performed. Granzyme B expression in OT-I cells in spleen (solid line), IEL (dashed line), and LPL (dotted line) was compared. The overlay histogram shows granzyme B expression in gated gfp-OT-I cells and isotype control (gray filled histogram). Representative histograms of 3–6 experiments performed (3–5 mice per experiment). MFI, mean fluorescence intensity; SPL, spleen; PEC, peritoneal exudate cell; IEL, intraepithelial lymphocytes; LPL, lamina propria lymphocytes.
In contrast to memory SPL OT-I, relatively few IEL OT-I cells produced interleukin (IL)-2 on in vitro antigenic (SIINFEKL peptide) restimulation (Figure 5b and d). Most of the SPL OT-I produced proinflammatory interferon-γ (IFN-γ; Figure 5a and d), whereas only half (50%) of IEL OT-I produced this cytokine (Figure 5a and d). Recent data indicate that type 17 CD8+ T cells represent a response in defense against intracellular pathogens.31 However OT-I cells induced by gp96-Ig vaccination did not produce IL-17, systemically or in the mucosal compartment (Figure 5c). The data show that gp96-Ig leads to the accumulation of highly cytotoxic antigen-specific CD8+ T cells within the mucosa with a reduced ability to produce inflammatory cytokines when compared to splenic OT-I.
OT-I cells that migrated to the intestinal mucosa after gp96-Ig vaccination express IFN-γ and IL-2, but not IL-17. To detect intracellular cytokine protein accumulation, 5 days after IP NIH-3T3-OVA-gp96-Ig immunization, SPL, IEL, and LPL were incubated with (a) and (b) 20 nm SIINFEKL peptide and 10 ng ml−1 Brefeldin A for 5 h or with (c) 1 μg ml−1 ionomycin, 50 ng ml−15 phorbol 12-myristate 13-acetate, and 2 mm monensin. Cells were first stained for surface anti-CD8 and then fixed/permeabilized, and intracellular staining for IFN-γ, IL-2, or IL-17 was performed. Representative dot plots show gated CD8 cells expressing (a) IFN-γ, (b) IL-2, (c) IL-17, and (a–c) gfp-OT-I. Numbers in the quadrants represent percent positive cells within the CD8 gate. (d) Frequency of IFN-γ and IL-2 producing OT-I expressed as %±s.e.m. of total OT-I. The number of mice per group was n=3–6. *P<0.05, **P<0.01 (compared with SPL OT-I). SPL, spleen; IEL, intraepithelial lymphocytes; LPL, lamina propria lymphocytes; IFN-γ, interferon-γ; IL-2, interleukin 2.
Gp96-Ig immunization increases frequency of CD103+ (αEβ7) DCs and efficiently induces CCR9 on responding T cells in vitro
To activate CD8 and natural killer cells, secreted gp96-peptide complexes need to be taken up by DCs or peritoneal macrophages.17 It has been reported that CD103+ DCs are important for generation of gut-tropic CD8 effector cells12 as well as T regulatory cells.32 Also, it has been shown that CD103−/− mesenteric lymph node (MLN) DCs are as efficient as wild-type MLN DCs at inducing CCR9.13 We therefore analyzed CD11chighMHC class IIhigh cells from the PEC of control (phosphate-buffered saline, PBS) or vaccinated (3T3-OVA and 3T3-OVA-gp96) mice for their CD103 expression. Gp96-Ig immunization induced dramatic increase of CD11chighMHC class IIhighCD103+ cells compared to control mice (P<0.001) (Figure 6a and b; Supplementary Figure 2). To investigate whether peritoneal CD103+ cells are required for priming and induction of gut-homing receptors on OT-I cells, we co-cultured carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled OT-I cells with sorted, Ova peptide (pOva; 2 nm)-pulsed CD103+ and CD103− DCs from PEC of gp96 vaccinated or control mice (PBS). Expression of CCR9 on responding T cells was assessed 5 days later (Figure 6c and d). As expected, both subsets CD103+ and CD103− DCs from vaccinated and control animals induced proliferation of OT-I cells, but only CD103+ DCs induced CCR9 on responding OT-I cells (Figure 6c and d). CD103+ DCs isolated from gp96 vaccinated mice induced comparable levels of CCR9 compared with CD103+ DCs from control animals. We have recently shown28 that LTα (lymphotoxin-α)-deficient mice showed normal OT-I expansion in the PEC when compared with wild-type mice. This finding suggested that LNs are not essential for gp96-mediated peptide cross-priming and that local cross-priming takes place at the site of gp96 release. To determine whether priming for generation of gut-tropic T cells is occurring in the peritoneum as opposed to the intestinal LN, we adoptively transferred CFSE-labeled CD45.2+ OT-I cells into CD45.1+ wild-type recipient mice. Recipient mice were vaccinated with 3T3-OVA-gp96-Ig, and CCR9 expression on responding OT-I cells in PEC and mesenteric LNs was determined 2 and 4 days later (Figure 7). CD8 cells present in the PEC by day 2 showed significant increase in proliferation and CCR9 upregulation, whereas at the same time CD8 cells in mesenteric LNs did not proliferate and remained CCR9 negative (Figure 7a–c). By day 4, gp96-dependent proliferation by CD8 cells in the PC was very pronounced and significantly higher than in the LNs. (Figure 7a and b). Together, these results show remarkable properties of secreted gp96 to induce local, peritoneal activation/proliferation and migration of OT-I cells to the intestinal LP (Figure 7c).
Intraperitoneal immunization with secreted gp96-Ig increases frequency of CD103+ DCs and efficiently upregulates CCR9 on responding T cells in vitro. Mice received two million 3T3-ova-gp96-Ig, 3T3-OVA, or PBS. Peritoneal cells were harvested on days 0, 1, 2 and 5, and stained for CD11c, MHC class II and CD103. (a) Absolute numbers of CD11chigh+MHC class IIhigh+ cells expressing CD103 within PEC cells. Results are the mean±s.e. from three independent experiments (three mice per experiment). *P<0.05, **P<0.01, ***P<0.001 (compared with PBS). (b) Phenotypic characteristics of CD11chigh+MHCIIhigh+ cells from PEC 5 days after PBS or 3T3-OVA-gp96 vaccination. (c) Sorted CD103+ and CD103− DCs from vaccinated (3T3-OVA-gp96) and nonvaccinated (PBS) mice were pulsed with 2 nm SIINFEKL peptide and incubated with CFSE-labeled OT-I cells at the ratio 1:2. CCR9 on responding OT-I cells was assesses after 5 days by flow cytometry. Results are mean and s.e. from two experiments. *P<0.05, **P<0.01 (compared with CD103− DCs). (d) Plots are representative of two independent experiments. PEC, peritoneal cavity; CFSE, carboxyfluorescein diacetate succinimidyl ester; MHC, major histocompatibility complex; PBS, phosphate-buffered saline; DC, dendritic cell.
Priming for generation of gut-tropic T cells after gp96 vaccination is occurring in the peritoneum. CD45.1+ mice received two million 3T3-OVA-gp96-Ig IP 2 days after CFSE-labeled CD45.2+OT-I transfer (one million) IV. Peritoneal and mesenteric lymph node (LN) cells were harvested on days 2 and 4, and stained for CD45.2, CD8, and CCR9. (a) Representative dot plot/histograms of CCR9 expression and of CFSE dilution on gated CD45.2+CD8T cells. CFSE profile of OT-I cells in the PEC in the absence of gp96 vaccination shown in gray filled histograms (b) Line graph shows the percentage of transferred CFSE-labeled CD45.2+OT-I cells in MLN and PEC at days 2 and 4 that have undergone 0–8 cell divisions as calculated by FlowJo curve-fitting software. Results are mean and s.e. from two experiments. *P<0.05, **P<0.01, ***P<0.001 (compared with MLN). (c) Bars represent mean±s.e. of the percentage CCR9+ OT-I cells (n=3). ***P<0.001 (compared with MLN). (d) Schematic representation of gp96-Ig-induced activation/proliferation and migration of OT-I cells to the intestinal lamina propria after intraperitoneal immunization. PEC, peritoneal cavity; MLN, mesenteric lymph nodes; DC, dendritic cell; CFSE, carboxyfluorescein diacetate succinimidyl ester.
The IP route of immunization is most effective for generating mucosal immunity
The physiological microenvironment of inductive sites with its characteristic resident populations, including macrophages, DCs, epithelial cells, B and T cells imprints the ensuing lymphocyte immune responses. Effector T cells generated in different lymphoid organs show distinct tissue tropisms, a feature that appears to be regulated by an organ-specific induction of adhesion molecules and chemokine receptors during T-cell priming. DCs are known to be the ‘key’ cells for imprinting of tissue-tropic T effector cells. Immunization with secreted gp96-Ig in the peritoneal environment seems to generate preferentially gut-tropic effector cells (Figures 4, 6, and 7). To compare different routes of immunization by gp96-Ig, we studied several vaccine priming sites (Figure 8). The IP route was twice as effective as the subcutaneous route for generating mucosal immunity (LPL and IEL) while generating equal systemic immunity (SPL). Intradermal immunization with gp96-Ig generated the same degree of splenic and LN OT-I but fewer LPL OT-I than IP immunization. Interestingly, intradermal immunization did not induce migration of OT-I to the intraepithelial compartment. In contrast to IP immunization, intrarectal and intravaginal immunization by instillation of gp96-Ig-secreting cells induced only mucosal immunity; a systemic response in the SPL was undetectable. This finding suggests that the generation of immune responses in certain location can be controlled by choosing appropriate vaccination routes.
Intraperitoneal immunization with secreted gp96-Ig stimulates more mucosal immunity compared with subcutaneous and intradermal stimulation, but similar systemic immunity; vaginal and rectal instillation of cells secreting gp96-Ig mediates only mucosal immunity. Mice received one million purified gfp-OT-I IV. After 2 days two million 3T3-OVA-gp96-Ig cells were injected by different routes: intraperitoneal, subcutaneous, or intradermal; for rectal and vaginal immunization gp96-Ig-secreting cells were instilled. After 5 days the frequency of gfp-OT-I was analyzed in spleen (SPL), mesenteric lymph nodes (MLNs), within intraepithelial lymphocytes (IEL), Peyer's patch lymphocytes (PPL), and lamina propria lymphocytes (LPL). Results are the mean and s.e. of 2–4 experiments (2–4 mice per group). *P<0.05, **P<0.01 (compared with intraperitoneal immunization).
Discussion
As gatekeepers of the immune response, DCs serve as the prime integrators of two pieces of environmental information—evidence for danger, e.g., inflammatory cytokines or microbial PAMP, and the antigenic signature of the pathogen. Although these are traditionally thought as two independent signals, extracellular gp96 packages both signals into a single complex targeting DCs. Gp96 itself activates and matures DCs and natural killer cells whereas the chaperoned peptide is cross-presented through MHC class I to CD8T cells. This dual function makes extracellular gp96-peptide complexes extraordinarily efficient in priming CTL responses. This communication documents that gp96-Ig-peptide complexes fulfill the same role in mucosal CD8 CTL activation, clonal expansion, and trafficking to mucosal membranes.
The gut mucosa is frequently exposed to pathogenic bacteria and viruses. In many epidemic diseases including typhoid fever, cholera, poliomyelitis and, most recently, HIV, the mucosa acts as the main port of entry. Secretory IgA and adaptive CD8+ IEL and LPL are clearly in a strategic location as early defenders against pathogenic attack to prevent invasion by pathogens or their spreading after initial infection.33 As to viral pathogens, a preventive vaccine must be able to generate mucosal immunity that neutralizes the virus or destroys infected cells before viral replication and spreading. Although virus neutralization is primarily achieved by antibody, killing of infected cells is the task primarily of adaptive CD8+ CTL. In particular intraepithelial, adaptive CD8+ IELs are in a frontline position to eradicate infected cells.
Our previous studies provided evidence that cell-secreted gp96-Ig vaccines provide powerful stimuli for the expansion and differentiation of antigen-specific CD8 CTL systemically and were effective as protective and therapeutic tumor vaccines16, 20, 28 (for review, see Podack and Raez34). Expanding the analysis to mucosal compartments, we now show that secreted, gp96-Ig-based, cellular vaccines also induce antigen-specific CD8 CTL in the intraepithelial and LP compartments of the gut mucosa and therefore may have important uses for the development of mucosal vaccines.
The adoptive transfer system using gfp-marked TCR transgenic CD8T cells (OT-I) was used to quantify antigen-specific mucosal memory CD8T cells present in relevant anatomic sites and determine their phenotype and functional properties. Previous studies by other groups have used ova-recombinant viruses and bacteria and adoptively transferred TCR transgenic OT-I cells to follow the distribution of effector cells following infection to nonlymphoid compartments in the periphery and intestinal space.2, 3, 35, 36, 37 The data indicated that memory responses of cells isolated from nonlymphoid tissue were maintained much longer than in the SPL. Similarly, memory responses in intestinal IEL were maintained for prolonged time periods. These data suggest that vaccines that are able to induce high levels of adaptive mucosal CD8 effector cells may provide long-lasting immunity against infection with the specific pathogen. In support of this, vaccine induced CD8+ CTLs present at mucosal sites of infection delayed mucosal CD4 depletion by simian/human immunodeficiency virus whereas systemic presence of specific CD8 CTL had little effect on mucosal CD4 depletion.38
The use of viruses and bacteria or of viral vectors or attenuated viruses for induction and analysis of the immune response relies, to a large extent, on the ability of viral or bacterial components to activate the immune system, e.g., through pattern recognition receptors. In addition, pathogen replication or vector replication may contribute to the observed effects of immunization. At the same time viral and bacterial pathogens tend to manipulate and deregulate the immune response to their own advantage to increase their own replication and survival. The outcome of the observed immune response thus is the result of complex interactions that are not readily dissected. In the modality of gp96-Ig immunization described here, cells express the relevant antigen (OVA), which is used as reporter antigen together with TCR transgenic reporter cells, similar to studies noted above. The same antigen-containing cells not secreting gp96-Ig are used as controls, restricting the observed immune response to the presence or absence of secreted gp96-Ig chaperoning the relevant peptides. Importantly, the use of allogeneic or syngeneic cells containing the antigen and secreting gp96-Ig elicited essentially the identical, antigen-specific immune response. This finding suggests that gp9-Ig-mediated immune activation is independent and possibly dominant over other immune responses, including allogeneic stimulation (NIH-3T3) or immune evasion by tumors (e.g., EG7—lymphoma).
Because the only difference between control and immune activation is the secretion of gp96-Ig, the observed immune response is entirely attributable to the adjuvant and cross-priming effect of gp96-peptide complexes, which has been studied in detail by several groups as described in the beginning. The IgG1-Fc tag used to replace the KDEL sequence in our study has no effect on the ensuing immune response as reported previously.22 The use of cells secreting gp96-Ig described here, rather than the use of purified soluble gp96 used by others, has major effects on the potency of the immune response. Cell-secreted gp96-Ig is 10–20 times more potent than an equivalent amount of soluble gp96-Ig.28 We interpret this finding to indicate that the immune system perceives continuous secretion and antigenic stimulation by gp96-Ig at the injection site as being equivalent to virus or bacterial replication, resulting in a vigorous immune responses. The data reported above are in support of this hypothesis and indicate that gp96-Ig-mediated mucosal (and systemic) immunity is quite comparable to the immune response induced by infectious agents.
Accordingly, we show that the IP immunization system, which relies on secreted heat shock fusion protein gp96-Ig, induced strong mucosal CD8-CTL responses in addition to systemic CTL responses. A major effect of gp96-Ig immunization was the rapid migration of OT-I into the LPL and intraepithelial compartments (IEL) (Figures 1 and 2). Immigrating cells were clearly identifiable by expression of high levels of CD44, CD103, CCR9, and α4β7 and downregulation of CD62L and CCR7 (Figure 3). Even though we did not follow up the phenotype of OT-I cells for long period, our data suggest that gp96-Ig-induced mucosal phenotypes had the general feature of gut memory. Furthermore, we found very high expression of the cytolytic mediator (granzyme B) in IEL OT-I and LPL OT-I (Figure 4b). The properties of gp96-Ig-induced mucosal CD8 CTL thus corresponded closely to those observed previously following lymphocytic choriomeningitis virus and vesicular stomatitis virus infection, including high granzyme B expression and low IL-2 and CD62L expression.29
Interestingly, it has been reported that in the absence of innate stimuli, or adjuvant, soluble OVA-activated OT-I IEL and LPL showed cytolytic activity, whereas splenic OT-I did not, suggesting that the gut environment provides an intrinsic stimulation, perhaps provided in part by the intestinal microflora.36 Moreover, although adoptively transferred OT-I CD8T cells actively migrated to the intestine and differentiated into cytolytic effector cells in response to systemic immunization with soluble OVA, long-lived memory CD8T cells were not generated.2 In contrast, recipient mice immunized with rVSV-OVA generated systemic as well as mucosal memory and both, mucosal and splenic OT-I CD8 memory T cells showed lytic activity, suggesting differences in the initial priming events between soluble OVA and rVSV-OVA were responsible for this discrepancy. Although nonviral and nonbacterial in nature, gp96-mediated mucosal and systemic CD8 CTL responses bear the hallmarks of memory responses characteristically seen after viral or bacterial infections. We attribute this observation to the adjuvanticity of gp96, which is specifically directed toward cross-priming cellular, cytotoxic CD8-CTL responses.21 In addition the continuous secretion of gp96-Ig from the injected cells for up to 5 days, may, as mentioned above, resemble viral replication and contribute to the cytotoxic response.
It is well appreciated that the site of immunization directs the imprinting of the ensuing T-cell response and controls the expression of trafficking molecules.39, 40 Our data indicate that the best route of vaccination for induction of both systemic and mucosal CD8-CTL immunity is the IP route in mice. Similar data are also emerging in nonhuman primates (unpublished data). Gp96-Ig immunization increases frequency of CD11chighMHC class IIhighCD103+ cells in PEC (Figure 6a). Phenotypic analysis of CD11chighMHC class IIhighCD103+ cells revealed that these cells are CD8 negative, express CD11b, CD40, CD80, CCR7, B220, and low levels of CD86 and Gr-1 (Supplementary Figure 2). CD103+CD11b+ and CD103+CD8− DC populations are more prominent in MLN and colonic LP.41 Recently, CD8−CD11bhighMHC classIIhighCD11chigh CD103+ DC population was found within omental tissue of normal healthy mice.42 These cells cross-present exogenous antigen for MHC class I-restricted T-cell responses. In the light of this finding and findings of Bedoui et al.43 that CD103+ DCs are the main migratory subtype with dominant cross-presenting ability, induction of CD103+ DCs by gp96 represents ideal vaccination strategy for priming effective immunity. Whether the priming after IP administration of gp96 occurs in the milky spots of the greater omentum remains to be determined.
Gp96-Ig-induced gut-migrating OT-I cells represent type a IEL, which express high amounts of granzyme B, but at the same time after in vitro peptide stimulation produce less IFN-γ and IL-2 than splenic OT-I (Figure 5). Also, IEL OT-I did not produce IL-17, a cytokine that has been associated with autoimmune diseases44 and virus-induced wasting disease in mice with CD8T cells that lack both T-bet and Eomes and overexpress IL-17.31 We suggest that antigen-specific IELs in the intestine respond to antigen stimulation with strong cytolytic activity and diminished cytokine secretion to prevent the development of intestinal immunopathology while eliminating infected cells.
In summary, gp96-Ig-induced antigen-specific CD8T cells have the ability to migrate to mucosal surfaces and provide immediate and enhanced protection at the most likely entry site of invading pathogens. Cell-secreted gp96-Ig immunization uses an internal danger signal, gp96, whose release by necrotic cell death sets off a cellular immune response directed at antigenic, chaperoned peptides. Use of this pathoimmunological mechanism seems ideally suited for vaccine purposes, including stimulation of cellular mucosal immunity.
Methods
Cell lines
EG7 were obtained from Dr M Bevan (University of Washington, Seattle, WA) and transfected with the bovine papilloma-derived vector pCMG-His containing gp96-Ig as described16 or with vector alone as control. NIH-3T3 cells were obtained from the ATCC.
Animals
C57Bl/6-CD45.2+ and C57Bl/6-CD45.1+ mice were obtained from Jackson Laboratories (Bar Harbor, ME). Gfp transgenic mice were obtained by kind permission of the producers. C57BL/6 OT-I mice (obtained from Dr M Bevan) express a transgenic TCR (vα2,vβ5.1.2) specific for the H-2Kb-restricted peptide (ova) amino acid 257-264 (SIINFEKL) derived from chicken OVA. Gfp mice were crossed to OT-I mice to generate gfp-OT-I in the animal facility at the University of Miami according to institutional guidelines. The progeny mice were screened by PCR for the expression of the ova-TCR gene and for gfp fluorescence. All mice were used at 6–12 weeks of age.
Purification and adoptive transfer of OT-I cells
Single-cell suspensions of splenocytes were obtained from gfp-OT-I mice and were depleted of red blood cells with ammonium chloride buffer. Gfp-OT-I cells were purified by positive selection using anti-CD8a magnetic microbeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. More than 95% of the purified cells were CD8+, as determined by flow cytometric analysis. After Vα2 and Vβ5.1/2 expressions on purified cells were quantified by flow cytometry, one million OT-I cells were adoptively transferred in C57Bl/6 mice in a volume of 0.03 ml PBS IV.
OT-I cells (CD45.2+, gfp negative) were labeled with CFSE according to the manufacturer instructions (Invitrogen, Paisley, UK) and 106 cells were injected IV into C57Bl/6-CD45.1+ recipient mice.
Immunization
Two days after adoptive transfer of one million gfp-OT-I or CFSE-labeled OT-I, 4 × 106 EG7-gp96-Ig or 2 × 106 NIH-3T3-OVA-gp96-Ig cells or control EG7 or NIH-3T3-OVA cells were injected IP in a volume of 0.5 ml PBS into recipient mice. After 5 days, cells were isolated from SPL, PEC, draining MLNs and the small intestine, and were subjected to further analysis.
Isolation of small intestinal lymphocytes
Small intestinal lymphocytes were isolated as described previously.45 In brief, the small intestine was cut from 0.5 cm below the stomach (from duodenum) to 1 cm above the cecum. IELs were isolated as follows; the small intestine was removed, flushed with calcium-magnesium-free buffer (10 × calcium/magnesium-free Hank's balanced salt solution (Gibco BRL), 10 × HEPES bicarbonate buffer (Sigma-Aldrich, St Louis, MO), 5% fetal bovine serum (Gibco BRL) and H2O), Peyer's patches were dissected, and the intestines were cut longitudinally and then into pieces (2–5 mm). Gut pieces were treated with 1.3 mm EDTA (Sigma-Aldrich) in calcium-magnesium-free/fetal bovine serum buffer (30 min at 37 °C, shaking at 200 r.p.m.). To isolate LPL, we then treated gut pieces with 100 U ml−1 collagenase VIII (Sigma-Aldrich) in 5% RPMI 1640, 2 mm MgCl2, 2 mm CaCl2 (60 min at 37 °C, shaking at 200 r.p.m.). Lymphocytes were purified on a 44/67% Percoll gradient (General Electric Healthcare, Piscataway, NJ; 800 × g at 20 °C for 20 min).
Antibodies and flow cytometric analysis
Single-cell suspensions were stained with 7-amino-actinomycin D, anti-CD3, anti-CD45.2, anti-B220, anti-CD8α, anti-CD8β, anti-CD45.2, anti-TCRαβ, anti-TCRγδ, anti-CD62L, anti-CD44, ant-CD11b, anti-CD11c, anti-F4/80, anti-MHC class II (A–E), anti-CD40, anti-CD80, anti-CD86, anti-CD103 (aEβ7), anti-α4β7, anti-CCR9, and anti-CCR7 antibodies (directly conjugated to fluorescein isothiocyanate, phycoerythrin, PE-Cy7, PerCP, APC, Pacific Blue, Pacific Orange (BD Pharmingen, San Jose, CA; eBioscience San Diego, CA; or R&D Systems, Minneapolis, MN). Four–seven parameter flow cytometric analysis was performed using a FacsCalibur and LSR II (BD Immunocytometry Systems, San Jose, CA) with FlowJo (Three Star, Ashland, OR) or DIVA (BD Biosciences, San Jose, CA) software.
Intracellular cytokine (IFN-γ, IL-2, and IL-17) and granzyme B staining
Gut lymphocytes were isolated as described above, stained for surface anti-CD8 and then fixed/permeabilized, and intracellular staining for granzyme B was performed. To enhance intracellular cytokine protein accumulation (IFN-γ and IL-2), we cultured gut lymphocytes with 20 nm SIINFEKL peptide and 10 ng ml−1 of Brefeldin A (Sigma-Aldrich) for 5 h. Before staining, all cells were treated with purified anti-mouse CD16/CD32 (Fc-γIII/II receptor; BD Pharmingen). Granzyme B staining was performed on freshly isolated gut lymphocytes. Intracellular staining for IFN-γ, IL-2, and Granzyme B was performed using the Cytofix/cytoperm kit in accordance with the manufacturer's directions (BD Pharmingen).
Gut lymphocytes were stimulated with 1 μg ionomycin, 50 ng ml−1 phorbol 12-myristate 13-acetate (Sigma-Aldrich), and 2 μm monensin (eBioscience) for 5 h at 37 °C. After surface staining for CD8 (BD Pharmingen), cells were fixed/permeabilized and intracellular staining for IL-17F (eBioscience) was performed. Samples were analyzed on an LSR II with DIVA software.
In vitro cell cultures
Purified CD8+ OT-I cells (CD8+ T-cell isolation kit; Miltenyi Biotec) were labeled with CFSE according to the manufacturer's instructions (Invitrogen). For CD103+ and CD103− purification, PEC cells from 3T3-OVA-gp96 or PBS-treated mice at day 5 were sorted using anti-CD11c, anti-CD11b, anti-MHC class II, anti- F4/80 and anti-CD103 on FACSAria (BD Biosciences). Before staining, cells were preincubated with Mouse BD Fc Block (BD Biosciences). DC fractions were 95–98% pure. DCs were pulsed with 2 nm SIINFEKL peptide for 1 h at 37 °C, washed extensively, and 105 DCs were incubated with 2 × 105 CFSE-labeled OT-I cells in the flat-bottom 96-well plates in 200 ml complete medium. The phenotype of responding OT-I cells was assessed by flow cytometry after 5 days.
Statistical analysis
Statistical analyses were performed using paired or unpaired two-tailed Student's t-test.
Disclosure
Dr Podack has financial interest in the technology reported.
References
Nagler-Anderson, C. Man the barrier! Strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 1, 59–67 (2001).
Kim, S.K., Schluns, K.S. & Lefrancois, L. Induction and visualization of mucosal memory CD8T cells following systemic virus infection. J. Immunol. 163, 4125–4132 (1999).
Masopust, D., Jiang, J., Shen, H. & Lefrancois, L. Direct analysis of the dynamics of the intestinal mucosa CD8T cell response to systemic virus infection. J. Immunol. 166, 2348–2356 (2001).
Masopust, D., Vezys, V., Marzo, A.L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Belyakov, I.M. & Berzofsky, J.A. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity 20, 247–253 (2004).
Kunkel, E.J. & Butcher, E.C. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16, 1–4 (2002).
Berlin, C. et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Cepek, K.L. et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 372, 190–193 (1994).
Wagner, N. et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 382, 366–370 (1996).
Schon, M.P. et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J. Immunol. 162, 6641–6649 (1999).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Johansson-Lindbom, B. et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 (2005).
Jaensson, E. et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205, 2139–2149 (2008).
Srivastava, P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 20, 395–425 (2002).
Arnold, D., Wahl, C., Faath, S., Rammensee, H.G. & Schild, H. Influences of transporter associated with antigen processing (TAP) on the repertoire of peptides associated with the endoplasmic reticulum-resident stress protein gp96. J. Exp. Med. 186, 461–466 (1997).
Yamazaki, K., Nguyen, T. & Podack, E.R. Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J. Immunol. 163, 5178–5182 (1999).
Binder, R.J., Han, D.K. & Srivastava, P.K. CD91: a receptor for heat shock protein gp96. Nat. Immunol. 1, 151–155 (2000).
Matzinger, P. & Bevan, M.J. Induction of H-2-restricted cytotoxic T cells: in vivo induction has the appearance of being unrestricted. Cell Immunol. 33, 92–100 (1977).
Arnold, D., Faath, S., Rammensee, H. & Schild, H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J. Exp. Med. 182, 885–889 (1995).
Oizumi, S. et al. Surmounting tumor-induced immune suppression by frequent vaccination or immunization in the absence of B cells. J. Immunother. 31, 394–401 (2008).
Ramirez, S.R. et al. Glycoprotein 96-activated dendritic cells induce a CD8-biased T cell response. Cell Stress Chaperones 10, 221–229 (2005).
Strbo, N., Oizumi, S., Sotosek-Tokmadzic, V. & Podack, E.R. Perforin is required for innate and adaptive immunity induced by heat shock protein gp96. Immunity 18, 381–390 (2003).
Vabulas, R.M. et al. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 277, 15107–15112 (2002).
Berwin, B. et al. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 22, 6127–6136 (2003).
Binder, R.J., Anderson, K.M., Basu, S. & Srivastava, P.K. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J. Immunol. 165, 6029–6035 (2000).
Singh-Jasuja, H. et al. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur. J. Immunol. 30, 2211–2215 (2000).
Berwin, B., Hart, J.P., Pizzo, S.V. & Nicchitta, C.V. Cutting edge: CD91-independent cross-presentation of GRP94(gp96)-associated peptides. J. Immunol. 168, 4282–4286 (2002).
Oizumi, S., Strbo, N., Pahwa, S., Deyev, V. & Podack, E.R. Molecular and cellular requirements for enhanced antigen cross-presentation to CD8 cytotoxic T lymphocytes. J. Immunol. 179, 2310–2317 (2007).
Masopust, D., Vezys, V., Wherry, E.J., Barber, D.L. & Ahmed, R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8T cell population. J. Immunol. 176, 2079–2083 (2006).
Svensson, M. et al. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 1, 38–48 (2008).
Intlekofer, A.M. et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321, 408–411 (2008).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Sydora, B.C., Jamieson, B.D., Ahmed, R. & Kronenberg, M. Intestinal intraepithelial lymphocytes respond to systemic lymphocytic choriomeningitis virus infection. Cell Immunol. 167, 161–169 (1996).
Podack, E.R. & Raez, L.E. Allogeneic tumor-cell-based vaccines secreting endoplasmic reticulum chaperone gp96. Exp. Opin. Biol. Ther. 7, 1679–1688 (2007).
Kim, S.K., Reed, D.S., Heath, W.R., Carbone, F. & Lefrancois, L. Activation and migration of CD8T cells in the intestinal mucosa. J. Immunol. 159, 4295–4306 (1997).
Kim, S.K. et al. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Sci. USA 95, 10814–10819 (1998).
Pope, C. et al. Organ-specific regulation of the CD8T cell response to Listeria monocytogenes infection. J. Immunol. 166, 3402–3409 (2001).
Belyakov, I.M., Isakov, D., Zhu, Q., Dzutsev, A. & Berzofsky, J.A. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J. Immunol. 178, 7211–7221 (2007).
Belyakov, I.M., Hammond, S.A., Ahlers, J.D., Glenn, G.M. & Berzofsky, J.A. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J. Clin. Invest. 113, 998–1007 (2004).
Belyakov, I.M. et al. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95, 1709–1714 (1998).
Annacker, O. et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 202, 1051–1061 (2005).
Carlow, D.A., Gold, M.R. & Ziltener, H.J. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J. Immunol. 183, 1155–1165 (2009).
Bedoui, S. et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10, 488–495 (2009).
Zheng, Y. et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Laky, K., Lefrancois, L. & Puddington, L. Age-dependent intestinal lymphoproliferative disorder due to stem cell factor receptor deficiency: parameters in small and large intestine. J. Immunol. 158, 1417–1427 (1997).
Acknowledgements
This work was supported by Public Health Service Grants R21 AIO68515, R21/R33 AI073234, PO1 CA109094, and by a Grant from ACGT (Alliance for Cancer Gene Therapy). We thank James L Phillips from Flow Cytometry Core Facility, Silvester Cancer Center University of Miami for expert help.
Author information
Authors and Affiliations
Corresponding author
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/mi
Rights and permissions
About this article
Cite this article
Strbo, N., Pahwa, S., Kolber, M. et al. Cell-secreted Gp96-Ig-peptide complexes induce lamina propria and intraepithelial CD8+ cytotoxic T lymphocytes in the intestinal mucosa. Mucosal Immunol 3, 182–192 (2010). https://doi.org/10.1038/mi.2009.127
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2009.127
This article is cited by
-
Secreted heat shock protein gp96-Ig: next-generation vaccines for cancer and infectious diseases
Immunologic Research (2013)
-
The allure and peril of hematopoietic stem cell transplantation: overcoming immune challenges to improve success
Immunologic Research (2013)
-
Humanized mice: novel model for studying mechanisms of human immune-based therapies
Immunologic Research (2013)