Abstract
Interleukin (IL)-12 p35/p40 is a heterodimeric cytokine that plays an important role in T helper (Th) cell polarization and Th1 T-cell differentiation. Recent findings have shown that both p35 and p40 can form other cytokines with different proteins (IL-23: p19/p40; IL-35: p35/EBI3). Furthermore, the cytokine IL-27 (EBI3/p28) has been identified as a member of the IL-12 family. Here, we discuss the recent findings on the role of IL-12 family members in experimental colitis. In particular, the role of IL-23 as a master regulator of effector T-cell activation is highlighted. These findings have important implications for the design of new therapeutic approaches in chronic intestinal inflammation in humans.
Similar content being viewed by others
Introduction
There is increasing interest in the role of proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases (IBD; such as Crohn's disease (CD) and ulcerative colitis).1, 2, 3, 4, 5 IBDs are relapsing inflammations of the gastrointestinal tract not because of specific pathogens. Although ulcerative colitis primarily involves the colonic mucosa and submucosa and is marked by continuous inflammation of the large intestine, CD is characterized by transmural, segmental inflammation that for the most part involves the distal ileum and colon, but can potentially affect any part of the gastrointestinal tract as well.1 Although the precise etiology of both CD and ulcerative colitis is still unknown, recent data suggest that predisposing genetic factors (e.g., NOD2, OCTN, interleukin (IL)-23R, autophagy genes) and bacterial antigens lead to an unbalanced activation of the mucosal immune system that in turn causes chronic intestinal inflammation.6, 7, 8, 9 The present review will focus on the role of cytokine signaling in T cells and summarize some recent data on clinical studies in which these pathways were targeted in IBD patients. In particular, special attention will be paid to the role of IL-12 family members, as these cytokines seem to play a predominant role in IBD pathogenesis.
T-cell Cytokine Production in IBD
Mucosal T cells are a special subgroup of cells that is hyporesponsive to T-cell receptor stimulation.2 Therefore, mucosal T lymphocytes are critically dependent on costimulation. In IBD patients, T-cell activation seems to be driven by luminal antigens and antigen-presenting cells that are capable of producing cytokines. Although differentiated T cells in CD produce a T helper (Th)1-type cytokine profile, the cytokine profile in ulcerative colitis is characterized by the increased production of the Th2 cytokines IL-5 and IL-13.10, 11 However, IL-4 levels are decreased in ulcerative colitis and CD. In addition, tumor necrosis factor is produced by both Th1 and Th2 cells as well as by macrophages. In addition, Th3 and regulatory CD25+ CD4+ T cells seem to exist in the normal gut that produce transforming growth factor-β and IL-10, respectively.12, 13 Their contribution to the pathogenesis of human IBD is largely unknown, but it has been shown that these cells are capable to exert immunosuppressive functions when cultured ex vivo.14
CD4+ Th1 or Th2 cells seem to play a key pathogenic role in the pathogenesis of IBD. This idea is supported by the reported clinical effects of CD4+ T-cell depletion in patients with CD. Consistently, antibodies to tumor necrosis factor that are successfully used in treating CD patients have been shown to induce rapid mucosal T-cell apoptosis within 2 days indicating that the therapeutic efficacy of these antibodies could be because of the induction of T-cell apoptosis.15
To illuminate the reasons for the different Th cell polarization in IBD, numerous studies were conducted in recent years. Many studies have addressed the role of IL-12 family members in IBD. This family of cytokines now comprises several members of heterodimeric proteins, including IL-12 p35/p40, IL-23 p19/p40, IL-27 EBI3 (Epstein–Barr virus-induced gene 3)/p28, and IL-35 p35/EBI3.16 The IL-12 heterodimer (p35/p40) produced by CD8α dendritic cells or macrophages is a pivotal cytokine that controls Th1 T-cell differentiation; a function that involves activation and phosphorylation of the transcription factor signal transducer and activator of transcription 4 (STAT4).16, 17, 18, 19, 20 It has been shown that Th1-mediated animal models of IBD are associated with increased IL-12 production and neutralizing antibodies to IL-12 have been shown to suppress Th1-mediated chronic intestinal inflammation in mice by the prevention of Th1 T cell-activation and the induction of Fas-mediated T-cell apoptosis.21, 22, 23, 24 STAT4-deficient T cells showed reduced capacity to induce Th1-mediated colitis in an adoptive transfer system, whereas STAT4-transgenic mice develop Th1-mediated colitis upon immunization. However, it is not clear, whether the effects of STAT4 in vivo are related to IL-12, as IL-23 (p19/p40) has been recently shown to activate STAT4 in T cells.25, 26 Indeed, using p19- and p35-deficient mice, Uhlig et al.27 observed that IL-23 but not IL-12 is required for colonic inflammation, whereas IL-12 rather than IL-23 controls systemic weight loss upon administration of agonistic CD40 antibodies. Taken together, these data indicate an important function of IL-23 for local initiation of T-cell-independent gut inflammation rather than systemic inflammation. In addition to its effects on Th17 cells in chronic intestinal inflammation,28 IL-23, thus, may directly activate cells of the mucosal immune system such as macrophages to produce proinflammatory cytokines and emerges as an attractive therapeutic target both in T-–cell-dependent and -independent colitis. However, IL-23 has been recently shown to crossregulate IL-12 production during initiation of T-cell-dependent 2,4,6-trinitrobenzenesulfonic acid colitis, and may thereby prevent early Th1 T-cell responses in colitis (Figures 1 and 2).29 Thus, IL-23 seems to be a pleiotropic cytokine in various models of intestinal inflammation. It seems that IL-23 has a more prominent role in the mucosal immune system than in systemic immune responses by exerting direct effects on both T cells and antigen-presenting cells in the gut. The data by Uhlig and co-workers27 suggest that IL-23 may induce gut inflammation even in the absence of T cells and implicate new avenues for T-cell-independent therapy of IBD. On the basis of the recent discovery of IL-23R mutations in IBD patients,8 it is tempting to speculate that such mutations could be used to predict responsiveness toward anti-IL-23 or anti-IL-12/IL-23 p40 antibody therapy in the future. The potential clinical relevance of these findings is underlined by the observation that anti-IL-12/IL-23 p40 antibodies showed clinical efficacy in a pilot trial in active CD.30
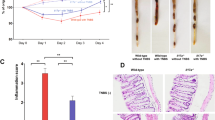
High-resolution miniendoscopy in mice with 2,4,6-trinitrobenzenesulfonic acid colitis (adapted from ref. 29). IL-23 p19-deficient mice showed more severe colitis than wild-type mice (left panels). Furthermore, anti-IL-12 p40 antibody therapy protected mice from such colitis (right panels).
Cytokine signaling by IL-12 and IL-23 in T lymphocytes. Both cytokines share the p40 subunit.
IL-27 has been identified as a new bioactive member of the IL-12 cytokine family.31, 32, 33, 34, 35, 36 It consists of an IL-12 p40-related polypeptide, denoted EBI3 and a new p28 subunit with some similarities to IL-12 p35 and IL-23 p19, respectively. Over the last years, IL-27 has emerged as a pivotal cytokine in the adaptive immune system by controlling T-cell-dependent immune responses. Hereby, IL-27 activates the transcription factors STAT1 and STAT3 in naive CD4 T cells and NK cells. Although STAT1 phosphorylation is required for IL-27-mediated activation of the Th1 master transcription factor T box protein expressed in T cells (T-bet), STAT3 is considered to be important for IL-27-induced T-cell proliferation. However, the role of IL-27 in IBD remains to be investigated. This also holds true for IL-35, a very recently identified cytokine that controls activation of regulatory T lymphocytes. However, p35 and EB13 deficient T cells failed to cure experimental colitis suggesting that IL-35 controls Treg function.
In 2000, a transcription factor denoted T-bet has been identified as a factor that controls IFN-γ production and Th1 development.37, 38, 39 The key role of T-bet in regulating IFN-γ production in Th1 cells is supported by studies showing profound suppression of IFN-γ production and IL-12Rβ2 chain expression in CD4+ T lymphocytes from T-bet-deficient mice. The potential clinical importance of this finding is shown by the fact that T-bet-deficient T cells fail to induce Th1-mediated experimental colitis in the CD4+ CD62L+-adoptive transfer model of colitis.40 As CD in humans is associated with strongly increased expression of T-bet in lamina propria T cells,40 these data provide a rationale for selective targeting of this transcription factor in this disease. Such targeting has the potential advantage of targeting the functional activity of various proinflammatory cytokines, simultaneously, rather than of a single cytokine (as in the case of neutralizing anti-cytokine antibodies). However, it should be noted that T-bet in antigen-presenting cells plays a protective role in colitis.41
Taken together, much progress has been made in understanding the immunopathogenesis of IBD. Various studies point toward an important role of cytokine signaling by IL-12 family members in experimental colitis and IBD patients. These data provide a rationale for the design of new therapeutic strategies in IBD in the near future. In particular, anti-IL-12/IL-23 p40 and anti-IL-23 p19 strategies should be considered.
Disclosure
Markus F. Neurath has received consulting and lecture fees from Abbott, Shire, Giuliani, Essex, and Centocor.
References
Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 325, 928–937 (1991).
Duchmann, R. & Zeitz, M. Crohn's disease. In Mucosal Immunology 2nd edn (Ogra, P.L., Mestecky, J., Lamm, M., Strober, W., Bienenstock, J. & McGhee, J.R. eds) 1055–1080 ( Academic Press, San Diego, 1999).
Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 347, 417–429 (2002).
Wirtz, S. & Neurath, M.F. Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int. J. Colorectal. Dis. 15, 144–160 (2000).
Strober, S., Fuss, I.J. & Blumberg, R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunology. 20, 495–549 (2002).
Parkes, M. et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat. Genet. 39, 830–832 (2007).
Massey, D. & Parkes, M. Common pathways in Crohn's disease and other inflammatory diseases revealed by genomics. Gut 56, 1489–1492 (2007).
Duerr, R.H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Neurath, M.F. IL-23: a master regulator in Crohn disease. Nat. Med. 13, 26–28 (2007).
Fuss, I. et al. Disparate CD4+ lamina propria (LP) lymphocyte secretion profiles in inflammatory bowel disease. J. Immunol. 157, 1261–1270 (1996).
Fuss, I.J. et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 113, 1490–1497 (2004).
Powrie, F., Carlino, J., Leach, M.W., Mauze, S. & Coffman, R.L. A critical role for transforming growth factor-beta but not interleukin-4 in the suppression of T helper type 1-mediated colitis by CD45Rb (low) CD4+ T cells. J. Exp. Med. 183, 2669–2674 (1996).
Weiner, H.L. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 3, 947–954 (2001).
Maul, J. et al. Peripheral and intestinal regulatory CD4+ CD25 (high) T cells in inflammatory bowel disease. Gastroenterology 128, 1868–1878 (2005).
Van den Brande, J.M. et al. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn's disease. Gut 56, 509–517 (2007).
Goriely, S., Neurath, M.F. & Goldman, M. How microorganisms tip the balance between interleukin-12 family members. Nat. Rev. Immunol. 8, 81–86 (2008).
Magram, J. et al. IL-12-deficient mice are defective in IFN-gamma production and type 1 cytokine responses. Immunity 4, 471–481 (1996).
Moser, M. & Murphy, K.M. Dendritic cell regulation of TH1–TH2 development. Nat. Immunol. 1, 199–205 (2000).
Szabo, S.J., Jacobson, N.G., Dighe, A.S., Gubler, U. & Murphy, K.M. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity 2, 665–675 (1995).
O’Shea, J.J. Jaks, STATs, cytokine signal transduction, and immunoregualtion: are we there yet? Immunity 7, 1–11 (1997).
Neurath, M.F., Fuss, I., Kelsall, B.L., Stuber, E. & Strober, W. Antibodies to IL-12 abrogate established experimental colitis in mice. J. Exp. Med. 182, 1280–1289 (1995).
Neurath, M.F., Finotto, S., Fuss, I., Boirivant, M., Galle, P.R. & Strober, W. Regulation of T-cell apoptosis in inflammatory bowel disease: to die or not to die, that is the mucosal question. Trends Immunol. 22, 21–26 (2001).
Fuss, I.J., Marth, T., Neurath, M.F., Pearlstein, G.R., Jain, A. & Strober, W. Anti-interleukin 12 treatment regulates apoptosis of Th1T cells in experimental colitis in mice. Gastroenterology 117, 1078–1088 (1999).
Davidson, N.J., Hudak, S.A., Lesley, R.E., Menon, S., Leach, M.W. & Rennick, D.M. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J. Immunol. 161, 3143–3149 (1998).
Oppmann, B. et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biologic activities similar as well as distinct from IL-12. Immunity 13, 715–725 (2000).
Wiekowski, M.T. et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 166, 7563–7570 (2001).
Uhlig, H.H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Yen, D. et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116, 1310–1316 (2006).
Becker, C. et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J. Immunol. 177, 2760–2764 (2006).
Mannon, P.J., et al. & Anti-IL-12 Crohn's Disease Study Group. Anti-interleukin-12 antibody for active Crohn's disease. N. Engl. J. Med. 351, 2069–2079 (2004).
Pflanz, S. et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 16, 779–790 (2002).
Devergne, O. et al. A novel interleukin-12 p40-related protein induced by latent Epstein–Barr virus infection in B lymphocytes. J. Virol. 70, 1143–1153 (1996).
Pflanz, S. et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172, 2225–2231 (2004).
Neurath, M.F., Finotto, S. & Glimcher, L.H. The role of Th1/Th2 polarization in mucosal immunity. Nat. Med. 8, 567–573 (2002).
Kamiya, S., Owaki, T., Morishima, N., Fukai, F., Mizuguchi, J. & Yoshimoto, T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J. Immunol. 173, 3871–3877 (2004).
Wirtz, S. et al. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. J. Immunol. 174, 2814–2824 (2005).
Szabo, S.J., Kim, S.T., Costa, G.L., Zhang, X., Fathman, C.G. & Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655–669 (2000).
Szabo, S.J., Sullivan, B.M., Stemmann, C., Satoskar, A.R., Sleckman, B.P. & Glimcher, L.H. T-bet is essential for Th1 lineage commitment and IFN-gamma production in CD4 but not CD8T cells. Science 295, 338–342 (2002).
Mullen, A.C. et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 292, 1907–1910 (2001).
Neurath, M.F. et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J. Exp. Med. 195, 1129–1143 (2002).
Garrett, W.S. et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131, 33–45 (2007).
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Neurath, M. IL-12 family members in experimental colitis. Mucosal Immunol 1 (Suppl 1), S28–S30 (2008). https://doi.org/10.1038/mi.2008.45
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2008.45
This article is cited by
-
IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting
Nature Reviews Gastroenterology & Hepatology (2019)
-
IL-35: a new immunomodulator in autoimmune rheumatic diseases
Immunologic Research (2018)
-
Striking the right immunological balance prevents progression of tuberculosis
Inflammation Research (2017)
-
Interleukin-35 (IL-35) inhibits proliferation and promotes apoptosis of fibroblast-like synoviocytes isolated from mice with collagen-induced arthritis
Molecular Biology Reports (2016)