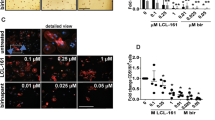
Abstract
The growth and survival of myeloma cells is critically regulated by cells of the bone marrow microenvironment, including osteoblasts. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) is a potent inducer of myeloma cell apoptosis, however, this antimyeloma activity is inhibited by osteoprotegerin (OPG) released from osteoblasts. Therefore, we hypothesized that specific agonists of TRAIL death receptors would not be inhibited by OPG released from osteoblasts and thus may represent a novel therapeutic approach in multiple myeloma. In the present study, TRAIL-induced apoptosis was demonstrated to be mediated through both DR4 and DR5. Specific agonist antibodies to DR4 or DR5 dose-dependently induced myeloma cell apoptosis, which was not prevented by OPG or by medium conditioned by osteoblasts. Co-culture of myeloma cells with osteoblasts protected against TRAIL-induced apoptosis of myeloma cells, and this protective effect was due to OPG. In contrast, the co-culture of myeloma cells with osteoblasts had no protective effect on apoptosis induced by specific agonists of DR4 or DR5. TRAIL has been proposed as a potential antitumour therapy, but within the bone marrow microenvironment OPG may interfere with the action of TRAIL. Specific agonists of TRAIL death receptors would not be subject to this inhibition and thus may provide an alternative specific antimyeloma therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Silvestris F, Cafforio P, Tucci M, Grinello D, Dammacco F . Upregulation of osteoblast apoptosis by malignant plasma cells: a role in myeloma bone disease. Br J Haematol 2003; 122: 39–52.
Yagita H, Takeda K, Hayakawa Y, Smyth MJ, Okumura K . TRAIL and its receptors as targets for cancer therapy. Cancer Sci 2004; 95: 777–783.
Mitsiades N, Mitsiades CS, Poulaki V, Anderson KC, Treon SP . Concepts in the use of TRAIL/Apo2L: an emerging biotherapy for myeloma and other neoplasias. Expert Opin Invest Drugs 2001; 10: 1521–1530.
Mitsiades CS, Treon SP, Mitsiades N, Shima Y, Richardson P, Schlossman R . TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood 2001; 98: 795–804.
Lincz L, Yeh T-X, Spencer A . TRAIL-induced eradication of primary tumour cells from multiple myeloma patient bone marrows is not related to TRAIL receptor expression or prior chemotherapy. Leukemia 2001; 15: 1650–1657.
Gazitt Y . TRAIL is a potent induced of apoptosis in myeloma cells derived from multiple myeloma patients and is not cytotoxic to hematopoietic stem cells. Leukemia 1999; 13: 1817–1824.
Kimberley FC, Screaton GR . Following a TRAIL: update on a ligand and its five receptors. Cell Res 2004; 14: 359–372.
Atkins GJ, Bouralexis S, Evdokiou A, Hay S, Labrinidis A, Zannettino AC et al. Human osteoblasts are resistant to Apo2L/TRAIL-mediated apoptosis. Bone 2002; 31: 448–456.
Daniels RA, Turley H, Kimberley FC, Liu XS, Mongkolsapaya J, Ch'En P et al. Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res 2005; 15: 430–438.
Kim K, Fisher MJ, Xu SQ, el-Deiry WS . Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res 2000; 6: 335–346.
Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem 1998; 273: 14363–14367.
Nyambo R, Cross N, Lippitt J, Holen I, Bryden G, Hamdy FC et al. Human bone marrow stromal cells protect prostate cancer cells from TRAIL-induced apoptosis. J Bone Miner Res 2004; 19: 1712–1721.
Shipman CM, Croucher PI . Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res 2003; 63: 912–916.
Shipman CM, Croucher PI, Russell RG, Helfrich MH, Rogers MJ . The bisphosphonate incadronate (YM175) causes apoptosis of human myeloma cells in vitro by inhibiting the mevalonate pathway. Cancer Res 1998; 58: 5294–5297.
Tinhofer I, Biedermann R, Krismer M, Crazzolara R, Greil R . A role of TRAIL in killing osteoblasts by myeloma cells. FASEB J 2006; 20: 759–761.
Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res 2006; 12: 1221–1228.
Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J et al. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 2003; 102: 1064–1069.
Lai FP, Cole-Sinclair M, Cheng WJ, Quinn JM, Gillespie MT, Sentry JW et al. Myeloma cells can directly contribute to the pool of RANKL in bone bypassing the classic stromal and osteoblast pathway of osteoclast stimulation. Br J Haematol 2004; 126: 192–201.
Giuliani N, Colla S, Morandi F, Barille-Nion S, Rizzoli V . Lack of receptor activator of nuclear factor-kB ligand (RANKL) expression and functional production by human multiple myeloma cells. Haematologica 2005; 90: 275–278.
Farrugia AN, Atkins GJ, To LB, Pan B, Horvath N, Kostakis P et al. Receptor activator of nuclear factor-kappaB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res 2003; 63: 5438–5445.
Sezer O, Heider U, Jakob C, Zavrski I, Eucker J, Possinger K et al. Immunocytochemistry reveals RANKL expression of myeloma cells. Blood 2002; 99: 4646–4647; author reply 4647.
Heider U, Langelotz C, Jakob C, Zavrski I, Fleissner C, Eucker J et al. Expression of receptor activator of nuclear factor kappaB ligand on bone marrow plasma cells correlates with osteolytic bone disease in patients with multiple myeloma. Clin Cancer Res 2003; 9: 1436–1440.
Seidel C, Hjertner Ø, Abildgaard N, Heickendorff L, Hjorth M, Westin J et al. Serum osteoprotegerin levels are reduced in patients with multiple myeloma with lytic bone disease. Blood 2001; 98: 2269–2271.
Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barillé S . Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood 2001; 98: 3527–3533.
Chuntharapai A, Dodge K, Grimmer K, Schroeder K, Marsters SA, Koeppen H et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J Immunol 2001; 166: 4891–4898.
Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med 2001; 7: 954–960.
Green DR, Kroemer G . Pharmacological manipulation of cell death: clinical applications in sight? J Clin Invest 2005; 115: 2610–2617.
Menoret E, Gomez-Bougie P, Geffroy-Luseau A, Daniels S, Moreau P, Le Gouill S et al. Mcl-1L cleavage is involved in TRAIL-R1- and TRAIL-R2-mediated apoptosis induced by HGS-ETR1 and HGS-ETR2 human mAbs in myeloma cells. Blood 2006; 108: 1346–1352.
Acknowledgements
This research was supported by the Leukaemia Research Fund. We are grateful to Dr Elizabeth Offord Cavin (Department of Nutrition, Nestle Research Center, Lausanne, Switzerland) for providing us with the hPOB-tert cell line.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Locklin, R., Croucher, P., Russell, R. et al. Agonists of TRAIL death receptors induce myeloma cell apoptosis that is not prevented by cells of the bone marrow microenvironment. Leukemia 21, 805–812 (2007). https://doi.org/10.1038/sj.leu.2404518
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404518