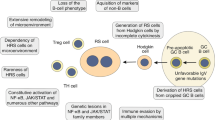
Abstract
Clonally related composite lymphomas of Hodgkin's lymphoma (HL) and Non-Hodgkin's lymphoma (NHL) represent models to study the multistep transformation process in tumorigenesis and the development of two distinct tumors from a shared precursor. We analyzed six such lymphomas for transforming events. The HLs were combined in two cases with follicular lymphoma (FL), and in one case each with B-cell chronic lymphocytic leukemia, splenic marginal zone lymphoma, mantle cell lymphoma (MCL) and diffuse large B-cell lymphoma (DLBCL). In the HL/FL and HL/MCL combinations, BCL2/IGH and CCND1/IGH translocations, respectively, were detected in both the HL and NHL. No mutations were found in the tumor suppressor genes FAS, NFKBIA and ATM. The HL/DLBCL case harbored clonal replacement mutations of the TP53 gene on both alleles exclusively in the DLBCL. In conclusion, we present the first examples of molecularly verified IgH-associated translocations in HL, which also show that BCL2/IGH or CCND1/IGH translocations can represent early steps in the pathogenesis of composite HL/FL or HL/MCL. The restriction of the TP53 mutations to the DLBCL in the HL/DLBCL case exemplifies a late transforming event that presumably happened in the germinal center and affected the fate of a common lymphoma precursor cell towards development of a DLBCL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rosenquist R, Menestrina F, Lestani M, Küppers R, Hansmann ML, Bräuninger A . Indications for peripheral light-chain revision and somatic hypermutation without a functional B-cell receptor in precursors of a composite diffuse large B-cell and Hodgkin's lymphoma. Lab Invest 2004; 84: 253–262.
Rosenquist R, Roos G, Erlanson M, Küppers R, Bräuninger A, Hansmann ML . Clonally related splenic marginal zone lymphoma and Hodgkin lymphoma with unmutated V gene rearrangements and a 15-yr time gap between diagnoses. Eur J Haematol 2004; 73: 210–214.
Tinguely M, Rosenquist R, Sundstrom C, Amini RM, Küppers R, Hansmann ML et al. Analysis of a clonally related mantle cell and Hodgkin lymphoma indicates Epstein–Barr virus infection of a Hodgkin/Reed-Sternberg cell precursor in a germinal center. Am J Surg Pathol 2003; 27: 1483–1488.
van den Berg A, Maggio E, Rust R, Kooistra K, Diepstra A, Poppema S . Clonal relation in a case of CLL, ALCL, and Hodgkin composite lymphoma. Blood 2002; 100: 1425–1429.
Küppers R, Sousa AB, Baur AS, Strickler JG, Rajewsky K, Hansmann ML . Common germinal-center B-cell origin of the malignant cells in two composite lymphomas, involving classical Hodgkin's disease and either follicular lymphoma or B-CLL. Mol Med 2001; 7: 285–292.
Marafioti T, Hummel M, Anagnostopoulos I, Foss HD, Huhn D, Stein H . Classical Hodgkin's disease and follicular lymphoma originating from the same germinal center B cell. J Clin Oncol 1999; 17: 3804–3809.
Bräuninger A, Hansmann ML, Strickler JG, Dummer R, Burg G, Rajewsky K et al. Identification of common germinal-center B-cell precursors in two patients with both Hodgkin's disease and non-Hodgkin's lymphoma. N Engl J Med 1999; 340: 1239–1247.
Küppers R, Zhao M, Hansmann ML, Rajewsky K . Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J 1993; 12: 4955–4967.
Küppers R . B cells under influence: transformation of B cells by Epstein–Barr virus. Nat Rev Immunol 2003; 3: 801–812.
Jungnickel B, Staratschek-Jox A, Bräuninger A, Spieker T, Wolf J, Diehl V et al. Clonal deleterious mutations in the IkappaBalpha gene in the malignant cells in Hodgkin's lymphoma. J Exp Med 2000; 191: 395–402.
Cabannes E, Khan G, Aillet F, Jarrett RF, Hay RT . Mutations in the IkBa gene in Hodgkin's disease suggest a tumour suppressor role for IkappaBalpha. Oncogene 1999; 18: 3063–3070.
Emmerich F, Meiser M, Hummel M, Demel G, Foss HD, Jundt F et al. Overexpression of I kappa B alpha without inhibition of NF-kappaB activity and mutations in the I kappa B alpha gene in Reed-Sternberg cells. Blood 1999; 94: 3129–3134.
Barth TF, Martin-Subero JI, Joos S, Menz CK, Hasel C, Mechtersheimer G et al. Gains of 2p involving the REL locus correlate with nuclear c-Rel protein accumulation in neoplastic cells of classical Hodgkin lymphoma. Blood 2003; 101: 3681–3686.
Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest 1997; 100: 2961–2969.
Montesinos-Rongen M, Roers A, Küppers R, Rajewsky K, Hansmann ML . Mutation of the p53 gene is not a typical feature of Hodgkin and Reed-Sternberg cells in Hodgkin's disease. Blood 1999; 94: 1755–1760.
Maggio EM, van den Berg A, de Jong D, Diepstra A, Poppema S . Low frequency of FAS mutations in Reed-Sternberg cells of Hodgkin's lymphoma. Am J Pathol 2003; 162: 29–35.
Maggio EM, Stekelenburg E, van den Berg A, Poppema S . TP53 gene mutations in Hodgkin lymphoma are infrequent and not associated with absence of Epstein–Barr virus. Int J Cancer 2001; 94: 60–66.
Müschen M, Re D, Bräuninger A, Wolf J, Hansmann ML, Diehl V et al. Somatic mutations of the CD95 gene in Hodgkin and Reed-Sternberg cells. Cancer Res 2000; 60: 5640–5643.
Gravel S, Delsol G, Al Saati T . Single-cell analysis of the t(14;18)(q32;q21) chromosomal translocation in Hodgkin's disease demonstrates the absence of this translocation in neoplastic Hodgkin and Reed-Sternberg cells. Blood 1998; 91: 2866–2874.
Miura I, Tamura A, Taniwaki M, Nakamura S, Nakamine H, Yoshino T et al. Detection of t(14; 18)(q32;q21) in hyperdiploid cells by fluorescence in situ hybridization in a patient with Hodgkin disease. Cancer Genet Cytogenet 2000; 123: 97–101.
Liberzon E, Avigad S, Yaniv I, Stark B, Avrahami G, Goshen Y et al. Molecular variants of the ATM gene in Hodgkin's disease in children. Br J Cancer 2004; 90: 522–525.
Takagi M, Tsuchida R, Oguchi K, Shigeta T, Nakada S, Shimizu K et al. Identification and characterization of polymorphic variations of the ataxia telangiectasia mutated (ATM) gene in childhood Hodgkin disease. Blood 2004; 103: 283–290.
Willis TG, Dyer MJ . The role of immunoglobulin translocations in the pathogenesis of B-cell malignancies. Blood 2000; 96: 808–822.
Gaidano G, Ballerini P, Gong JZ, Inghirami G, Neri A, Newcomb EW et al. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci USA 1991; 88: 5413–5417.
Volpe G, Vitolo U, Carbone A, Pastore C, Bertini M, Botto B et al. Molecular heterogeneity of B-lineage diffuse large cell lymphoma. Genes Chromosomes Cancer 1996; 16: 21–30.
Gronbaek K, Straten PT, Ralfkiaer E, Ahrenkiel V, Andersen MK, Hansen NE et al. Somatic Fas mutations in non-Hodgkin's lymphoma: association with extranodal disease and autoimmunity. Blood 1998; 92: 3018–3024.
Greiner TC, Moynihan MJ, Chan WC, Lytle DM, Pedersen A, Anderson JR et al. p53 mutations in mantle cell lymphoma are associated with variant cytology and predict a poor prognosis. Blood 1996; 87: 4302–4310.
Gumy-Pause F, Wacker P, Sappino AP . ATM gene and lymphoid malignancies. Leukemia 2004; 18: 238–242.
Stankovic T, Stewart GS, Byrd P, Fegan C, Moss PA, Taylor AM . ATM mutations in sporadic lymphoid tumours. Leuk Lymphoma 2002; 43: 1563–1571.
Noppe SM, Heirman C, Bakkus MH, Brissinck J, Schots R, Thielemans K . The genetic variability of the VH genes in follicular lymphoma: the impact of the hypermutation mechanism. Br J Haematol 1999; 107: 625–640.
Liu J, Johnson RM, Traweek ST . Rearrangement of the BCL-2 gene in follicular lymphoma. Detection by PCR in both fresh and fixed tissue samples. Diagn Mol Pathol 1993; 2: 241–247.
Graninger WB, Seto M, Boutain B, Goldman P, Korsmeyer SJ . Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest 1987; 80: 1512–1515.
Zoldan MC, Inghirami G, Masuda Y, Vandekerckhove F, Raphael B, Amorosi E et al. Large-cell variants of mantle cell lymphoma: cytologic characteristics and p53 anomalies may predict poor outcome. Br J Haematol 1996; 93: 475–486.
Villuendas R, Pezzella F, Gatter K, Algara P, Sanchez-Beato M, Martinez P et al. p21WAF1/CIP1 and MDM2 expression in non-Hodgkin's lymphoma and their relationship to p53 status: a p53+, MDM2-, p21-immunophenotype associated with missense p53 mutations. J Pathol 1997; 181: 51–61.
Nadel B, Marculescu R, Le T, Rudnicki M, Böcskör S, Jäger U . Novel insights into the mechanism of t(14;18)(q32;q21) translocation in follicular lymphoma. Leuk Lymphoma 2001; 42: 1181–1194.
Torlakovic E, Tierens A, Dang HD, Delabie J . The transcription factor PU.1, necessary for B-cell development is expressed in lymphocyte predominance, but not classical Hodgkin's disease. Am J Pathol 2001; 159: 1807–1814.
Re D, Müschen M, Ahmadi T, Wickenhauser C, Staratschek-Jox A, Holtick U et al. Oct-2 and Bob-1 deficiency in Hodgkin and Reed Sternberg cells. Cancer Res 2001; 61: 2080–2084.
Stein H, Marafioti T, Foss HD, Laumen H, Hummel M, Anagnostopoulos I et al. Down-regulation of BOB.1/OBF.1 and Oct2 in classical Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with immunoglobulin transcription. Blood 2001; 97: 496–501.
Schwering I, Bräuninger A, Klein U, Jungnickel B, Tinguely M, Diehl V et al. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood 2003; 101: 1505–1512.
Hell K, Lorenzen J, Fischer R, Hansmann ML . Hodgkin cells accumulate mRNA for bcl-2. Lab Invest 1995; 73: 492–496.
Liu YJ, Mason DY, Johnson GD, Abbot S, Gregory CD, Hardie DL et al. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur J Immunol 1991; 21: 1905–1910.
Garcia JF, Camacho FI, Morente M, Fraga M, Montalban C, Alvaro T et al. Hodgkin and Reed-Sternberg cells harbor alterations in the major tumor suppressor pathways and cell-cycle checkpoints: analyses using tissue microarrays. Blood 2003; 101: 681–689.
Tzankov A, Zimpfer A, Lugli A, Krugmann J, Went P, Schraml P et al. High-throughput tissue microarray analysis of G1-cyclin alterations in classical Hodgkin's lymphoma indicates overexpression of cyclin E1. J Pathol 2003; 199: 201–207.
Geng Y, Whoriskey W, Park MY, Bronson RT, Medema RH, Li T et al. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell 1999; 97: 767–777.
Müschen M, Rajewsky K, Krönke M, Küppers R . The origin of CD95-gene mutations in B-cell lymphoma. Trends Immunol 2002; 23: 75–80.
Koduru PR, Raju K, Vadmal V, Menezes G, Shah S, Susin M et al. Correlation between mutation in P53, p53 expression, cytogenetics, histologic type, and survival in patients with B-cell non-Hodgkin's lymphoma. Blood 1997; 90: 4078–4091.
Cho Y, Gorina S, Jeffrey PD, Pavletich NP . Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science 1994; 265: 346–355.
Acknowledgements
We are grateful to Julia Jesdinsky, Michaela Fahrig, Yvonne Blum and Kerstin Heise for excellent technical assistance. We thank Malcolm Taylor for helpful discussions. This work was supported by the Deutsche Krebshilfe, Mildred Scheel-Stiftung (70-3173-Tr3), the IFORES program of the University of Essen, Medical School, the Swiss National Science Foundation and stipends from the Swedish Society for Medical Research and the Werner-Gren Foundations to RR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu).
Supplementary information
Rights and permissions
About this article
Cite this article
Schmitz, R., Renné, C., Rosenquist, R. et al. Insights into the multistep transformation process of lymphomas: IgH-associated translocations and tumor suppressor gene mutations in clonally related composite Hodgkin's and non-Hodgkin's lymphomas. Leukemia 19, 1452–1458 (2005). https://doi.org/10.1038/sj.leu.2403841
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403841
Keywords
This article is cited by
-
Machine learning-based tissue of origin classification for cancer of unknown primary diagnostics using genome-wide mutation features
Nature Communications (2022)
-
Three coexisting lymphomas in a single patient: composite lymphoma derived from a common germinal center B-cell precursor and unrelated discordant lymphoma
International Journal of Hematology (2018)
-
A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors
Cancer Chemotherapy and Pharmacology (2014)
-
Rolle der Zytologie in der hämatopathologischen Diagnostik
Der Pathologe (2012)
-
Composite lymphoma in the anterior mediastinum: a case report and review of the literature
Diagnostic Pathology (2011)