Abstract
The enteric glial cells, in addition to being support structures for the enteric nervous system, have many other additional roles, such as modulators for the homeostasis of enteric neurons, cells involved in enteric neurotransmission and antigen-presenting cells. Moreover, in the last years, data have been accumulating that demonstrate a possible active role of these cells in the pathophysiology of gastrointestinal motor activity. Thus, as also shown by recent evidence in both experimental animal models, and in some human diseases, alterations of enteric glial cells might have some role in the development of intestinal motor abnormalities.
Similar content being viewed by others
Main
The enteric nervous system (ENS) is organized in a complex structure that controls motility, blood flow, uptake of nutrients, secretion, immunological and inflammatory processes in the gut.1 Two main cell populations are represented in the ENS, neurons and enteric glial cells (EGC), the latter being much more abundant (up to fourfold) than neurons.2
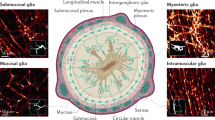
The old simplistic view of EGC, basically as support cells for the ganglia and/or nutritive elements for enteric neurons, has changed with the increase in knowledge, and it is now evident that these cells may have an important role in the economy of the digestive system. In fact, EGC display mechanical support, neurotransmitter, immune and homeostatic functions in the gut (Figure 1).3, 4 Even more intriguing is the putative role of EGC as players involved in gastrointestinal motility, as it will be discussed in this review.
EGC AND ABNORMAL GASTROINTESTINAL MOTILITY: EVIDENCE FROM HUMAN STUDIES
It has been pointed out that there is no gastrointestinal disorder for which an underlying glial defect has been established.3 However, it is possible that abnormalities relative to this cell population may have been overlooked.
EGC AND INTESTINAL INFLAMMATION
The concept that gastrointestinal motility is altered in mucosal inflammatory conditions of the gut is now well accepted, and studies in animal models clearly indicate a causal relationship between the presence of mucosal inflammation and altered motor function.5 Some intriguing experimental evidences have demonstrated that EGC may have a role in intestinal inflammatory processes,3, 6, 7 and that immune-mediated damage to enteric glia might participate in the initiation and/or the progression of inflammatory bowel disease.8 In fact, EGC functionally interact with lymphocytes,9, 10 respond actively to inflammation and become activated as antigen-presenting cells11 attracting immune cells to the ENS.7 Thus, it has been hypothesized that the EGC network disruption might represent an important cause for the development of inflammatory bowel diseases, especially Crohn's disease, even though human data are still lacking.7
On the other hand, looking at the above EGC functions and properties, it is not difficult to image an active role of these cells also in the pathogenesis of ‘functional’ gastrointestinal disorders. The latter are traditionally thought to occur in the absence of anatomical or biochemical abnormalities.12 However, studies in patients with irritable bowel syndrome demonstrated mild to moderate inflammatory infiltrates closely associated to the enteric plexuses and mucosal activation of the immune system,13, 14 and we have previously shown that some patients with severe intestinal dysmotility and megacolon may have an underlying myenteric ganglionitis (due to a prominent lymphoplasmacellular infiltrate within the myenteric plexus) responsible for their symptoms.15 Due to their properties and function, it is conceivable that EGC could play a role in these instances, by attracting immune cells to the ENS.3 However, such a hypothesis was not formally tested in these former studies.
Evidence for Involvement of ECG in Abnormal Gastrointestinal Motility
Only a few studies have been published in which the role of EGC has specifically investigated in human gastrointestinal motor disorders. For instance, we found a significant decrease of EGC (together with a decreased number of interstitial cells of Cajal (ICC)) in both the submucosal and myenteric plexuses in patients with colonic diverticular disease.16 Since in this condition the hypertrophied colonic smooth muscle may represent a partially obstructive mechanism, we hypothesized that the function and the population of EGC might be loss due, at least in part, to this mechanism, in analogy to what happens to ICC in similar experimental animal models.17
We have subsequently reported that the EGC (together with enteric neurons and ICC) are significantly reduced in patients with idiopathic severe slow-transit constipation requiring surgery for symptoms' relief18 (Figure 2a–d). The EGC decrease (but not that of ICC and enteric neurons) was also present in the small bowel (terminal ileum) of the same patients,19 suggesting that ECG might have some role in the abnormal motor activity described in these patients. Findings similar to those described above in the colons of patients with slow-transit constipation have recently been reported by our group in patients with chagasic and idiopathic megacolon.20
EGC (arrows) tightly packed around enteric neurons in a submucosal (a) and in a myenteric ganglion (b). S100 immunostaining, original magnifications × 40 (a) and × 100 (b). Full thickness specimen of human colon of a control subject (c) and of a patient with intractable slow-transit constipation (d). The arrows indicate EGC in the myenteric plexus. Note the rarefaction of these cells in (d). S100 immunostaining, original magnification × 10.
Moreover, we have found a significant decrease of EGC, but not ICC, in both the myenteric and submucosal plexuses of patients with severe constipation due to obstructed defecation refractory to treatment.21
PUTATIVE MECHANISMS FOR EGC INFLUENCE ON GASTROINTESTINAL MOTILITY: EVIDENCE FROM ANIMAL STUDIES
Only a few studies are available on this topic, mostly originating or being inferred from experimental animal models, and in which the motility data have frequently been reported as adjunct findings.
The development of both EGC and enteric neurons is strictly regulated by microenvironmental factors3, 22, 23 such as glial cell line-derived neurotrophic factor (GDNF), neutrophin-3 (NT-3), ciliary neurotrophic factor (CNTF) and leukemia inhibitory factor (LIF).3 Among these, GDNF has a prominent role in promoting the development and survival of enteric neurons, as suggested by the fact that the ENS almost completely fails to develop in GDNF knockout mice.24 The trophic function of GDNF is also supported by in vitro studies, demonstrating that this factor promotes the development of glia in unselected cell populations from fetal murine bowel.25 Moreover, it has been shown that mature EGC produce GDNF.26
So far, it has not been demonstrated that glial expression of trophic factors contributes to the maintenance of enteric neurons in man. However, several such lines of evidence are available for animal models. For instance, GDNF can modulate enteric neuronal survival and proliferation in postnatal mice through a neuropeptide Y-mediated mechanism,27 and GDNF overexpression prevents hyperglycemia-induced delayed gastric emptying in diabetic animals.28 Conversely, the absence of GDNF receptor alfa2 in mice causes the loss of substance P (an excitatory transmitter)-containing myenteric neurons, with subsequent decrease of small bowel transit.29
In addition, the selective ablation of EGC by a gliotoxin causes a decrease of small intestinal motility and transit in rats and, more importantly, this enteric glial dysfunction is not accompanied by intestinal inflammation.30 Overall, these data suggest that EGC are involved in the modulation of enteric neural pathways responsible for the control of the motor activity of the gut.
Glial cells are well recognized sources of neurotrophic factors and neurotrophins (both chief regulators of ontogenetic differentiation and adult function31, 32) in the central nervous system, and the latter control gene expression and neuronal phenotypes.33 Expression of neurotrophins (NT-3, -4, -5 and -6) and of their thyrosine receptor kinases has been described in the adult ENS,34, 35, 36 and there is evidence that these factors may be secreted by glial cells.37 Thus, it has been suggested that neurotrophins may be produced by EGC to modulate neuronal gene expression and in turn the phenotypes of enteric neurons.38 Interestingly, it has been reported recently that EGC may influence the neurochemical coding of enteric neurons. In fact, in a mouse model, enteric glial ablation caused a marked reduction in the vasoactive intestinal peptide and substance P immunoreactive neurons of the submucous plexus, with an increase of choline acetyltransferase and a decrease of nitric oxide synthase immunoreactive neurons in the myenteric plexus.39
Overall, the above and other evidences suggest a close functional link between EGC and enteric neurons.40
HYPOTHETICAL MECHANISMS OF GUT MOTOR ABNORMALITY RELATED TO EGC DYSFUNCTION IN HUMANS
How can we reconcile the clinicopathological observations in patients with the evidence obtained in experimental animal models? Although we are still unable to identify a human pathological process entirely due to abnormalities of EGC, it is likely that the significant decrease of this cell population described in several conditions (noteworthy, all characterized by constipation) may have pathophysiological implications. For instance, it could be hypothesized that the reduction of EGC, coupled to that of other cell types involved in the control of enteric motility, might have some pathogenetic role in the motor disturbances of these patients through several mechanisms. In fact, the decrease or loss of EGC might cause degeneration of enteric neurons due to the dysregulation of neurotrophic factors, in a manner similar to that observed in the animal models described above. With this respect, it is worth noting that patients with ‘idiopathic’ constipation and loss of EGC often have a concomitant loss of enteric neurons through increased apoptotic phenomena,18, 41 whereas in constipated patients with Alzheimer's disease (a degenerative condition of the central nervous system), EGC are preserved and the loss of enteric neurons is not due to apoptotic phenomena.42
Moreover, the loss of EGC in constipated patients is likely to aggravate the impaired pacemaker signals due to the decrease of ICC observed in these patients, since it has been postulated that ATP released from EGC provides a feedback system for ICC to modulate slow wave activity43 (Figure 3).
Unfortunately, we have yet no idea on why EGC (and ICC, and enteric neurons) are decreased in such patients; to date, the only evidence in an experimental animal model is that the number of EGC decreases with age.44 However, this influence has not been evaluated in human beings so far,45 and it was not confirmed in our age-matched controls.18 Moreover, we want to stress that the hypothesized damaging effect of anthraquinone laxatives on the ENS has not been confirmed with modern immunohistochemical techniques.46 Recently, we found chromosomal abnormalities of enteric neurons and EGC in severely constipated patients undergoing surgery and hypothesized that a genetic basis might be present to explain the decrease of these elements in the ENS in a subgroup of these subjects.47
CONCLUSIONS
It is probably safe to state that people interested in gastrointestinal motility should look in a different perspective at EGC. In fact, from a traditional, old-fashioned view of these cells as elements having a simple mechanical support function, hence no more than mere spectators of events, it can be hypothesized that the EGC might have a more active role than previously thought in the complex organization of the motor activity of the gastrointestinal tract. Further studies are obviously needed to confirm these preliminary observations (and speculations), to establish a more precise role for EGC (which interface between the neural and the immune systems) in the motor functions of the gut and, perhaps, to take the ‘idiopathic’ or the ‘functional’ out from the label of some disorders (ie, slow transit constipation).48 Moreover, in view of the new and exciting perspectives, such as the promises of neural stem cells transplantation49, 50, 51 for the treatment of disorders of the peripheral and central nervous system, these studies could possibly be helpful in establishing a more targeted therapeutic approach to some motor disorders of the gastrointestinal tract.52
CONFLICT OF INTEREST
None.
References
Goyal RK, Hirano I . The enteric nervous system. N Engl J Med 1996;334:1106–1115.
Jessen KR . Glial cells. Int J Biochem Cell Biol 2004;36:1861–1867.
Ruhl A . Glial cells in the gut. Neurogastroenterol Motil 2005;17:777–790.
Vasina V, Barbara G, Talamonti L, et al. Enteric neuroplasticity evoked by inflammation. Auton Neurosci 2006;126–127:264–272.
Collins SM . The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 1996;111:1693–1699.
Bush TG . Enteric glial cells. An upstream target for induction of necrotizing enterocolitis and Crohn's disease? Bioessays 2002;24:130–140.
Cabarrocas J, Savidge TC, Liblau RS . Role of enteric glial cells in inflammatory bowel disease. Glia 2003;41:81–93.
Cornet A, Savidge TC, Cabarrocas J, et al. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci USA 2001;98:13306–13311.
Geboes K, Rutgeerts P, Ectors N, et al. Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology 1992;103:439–447.
Ruhl A, Franzke S, Collins SM, et al. Interleukin-6 expression and regulation in rat enteric glial cells. Am J Physiol 2001;280:G1163–G1171.
Hirata I, Berrebi G, Austin LL, et al. Immunohistochemical characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon in inflammatory bowel disease. Dig Dis Sci 1986;31:593–603.
Drossman DA . What does the future hold for irritable bowel syndrome and the functional gastrointestinal disorders? J Clin Gastroenterol 2005;39(5 Suppl):S251–S256.
Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778–1783.
Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology 2002;123:1972–1979.
De Giorgio R, Barbara G, Stanghellini V, et al. Clinical and morphofunctional features of idiopathic myenteric ganglionitis underlying severe intestinal motor dysfunction: a study of three cases. Am J Gastroenterol 2002;97:2454–2459.
Bassotti G, Battaglia E, Bellone G, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol 2005;58:973–977.
Won KJ, Suzuki T, Hori M, et al. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil 2006;18:53–61.
Bassotti G, Villanacci V, Maurer CA, et al. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut 2006;55:41–46.
Bassotti G, Villanacci V, Cathomas G, et al. Enteric neuropathology of the terminal ileum in patients with intractable slow transit constipation. Hum Pathol 2006;37:1252–1258.
Iantorno G, Bassotti G, Kogan Z, et al. The enteric nervous system in chagasic and idiopathic megacolon. Am J Surg Pathol 2007;31:460–468.
Bassotti G, Villanacci V, Nascimbeni R, et al. Colonic neuropathological aspects in patients with intractable constipation due to obstructed defecation. Mod Pathol 2007;20:367–374.
Gershon MD, Rothman TP . Enteric glia. Glia 1991;4:195–204.
Young HM, Bergner AJ, Muller T . Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol 2003;456:1–11.
Sanchez MP, Silos-Santiago I, Frisen J, et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 1996;382:70–73.
Heuckeroth RO, Enomoto H, Grider JR, et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 1999;22:253–263.
Bar KJ, Facer P, Williams NS, et al. Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprung's disease. Gastroenterology 1997;112:1381–1385.
Anitha M, Chandrasekharan B, Salgado JR, et al. Glial-derived neurotrophic factor modulates enteric neuronal survival and proliferation through neuropeptide Y. Gastroenterology 2006;131:1164–1178.
Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest 2006;116:344–356.
Rossi J, Herzig KH, Voikar V, et al. Alimentary tract innervation deficits and dysfunction in mice lacking GDNF family receptor alfa2. J Clin Invest 2003;112:707–716.
Nasser Y, Fernandez E, Keenan CM, et al. The role of enteric glia in intestinal physiology: the effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol 2006;291:G912–G927.
Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci 1996;19:514–520.
Woolf CJ, Salter MW . Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765–1769.
Wirth MJ, Patz S, Wahle P . Transcellular induction of neuropeptide Y expression by NT4 and BDNF. Proc Natl Acad Sci USA 2005;102:3064–3069.
Hoehner JC, Wester T, Pahlman S, et al. Localization of neurotrophins and their high-affinity receptors during human enteric nervous system development. Gastroenterology 1996;110:756–767.
De Giorgio R, Arakawa J, Wetmore CJ, et al. Neurotrophin-3 and neurotrophin receptor immunoreactivity in peptidergic enteric neurons. Peptides 2000;21:1421–1426.
Lin A, Lourenssen S, Stanzel RD, et al. Nerve growth factor sensitivity is broadly distributed among myenteric neurons of the rat colon. J Comp Neurol 2005;490:194–206.
Giaroni C, De Ponti F, Cosentino M, et al. Plasticity in the enteric nervous system. Gastroenterology 1999;117:1438–1458.
Ruhl A . Glial regulation of neuronal plasticity in the gut: implications for clinicians. Gut 2006;55:600–602.
Aube AC, Cabarrocas J, Bauer J, et al. Changes in enteric neurone phenotype and intestinal functions in a transgenic mice model of enteric glia disruption. Gut 2006;55:630–637.
Murakami M, Ohta T, Otsuguro KI, et al. Involvement of prostaglandin E(2) derived from enteric glial cells in the action of bradykinin in cultured rat myenteric neurons. Neuroscience 2007;145:642–653.
Bassotti G, Villanacci V, Fisogni S, et al. Comparison of three methods to assess enteric neuronal apoptosis in patients with slow transit constipation. Apoptosis 2007;12:329–332.
Bassotti G, Villanacci V, Fisogni S, et al. Apoptotic phenomena are not a major cause of enteric neuronal loss in constipated patients with dementia. Neuropathology 2007;27:67–72.
Burnstock G, Lavin S . Interstitial cells of cajal and purinergic signalling. Auton Neurosci 2002;97:68–72.
Phillips RJ, Kieffer EJ, Powley TL . Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl) 2004;209:19–30.
Jill Saffrey M . Ageing of the enteric nervous system. Mech Ageing Develop 2004;125:899–906.
Villanacci V, Bassotti G, Cathomas G, et al. Is pseudomelanosis coli a marker of colonic neuropathy in severely constipated patients? Histopathology 2006;49:132–137.
Rossi E, Villanacci V, Fisogni S, et al. Chromosomal study of enteric glial cells and neurons by fluorescence in situ hybridization in slow transit constipation. Neurogastroenterol Motil 2007 (in press).
Bassotti G, Villanacci V . Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol 2006;12:4609–4613.
Galli R, Gritti A, Bonfanti L, et al. Neural stem cells: an overview. Circ Res 2003;92:598–608.
Burns AJ, Pasricha PJ, Young HM . Enteric neural crest-derived cells and neural stem cells: biology and therapeutic potential. Neurogastroenterol Motil 2004;16(Suppl. 1):3–7.
Rauch U, Hansgen A, Hagl C, et al. Isolation and cultivation of neuronal precursor cells from the developing human enteric nervous system as a tool for cell therapy in dysganglionosis. Int J Colorectal Dis 2006;21:554–559.
Rayner CK, Horowitz M . Gastrointestinal motility and glycemic control in diabetes: the chicken and the egg revisited? J Clin Invest 2006;116:299–302.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bassotti, G., Villanacci, V., Antonelli, E. et al. Enteric glial cells: new players in gastrointestinal motility?. Lab Invest 87, 628–632 (2007). https://doi.org/10.1038/labinvest.3700564
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3700564
Keywords
This article is cited by
-
Abnormal gut motility in inflammatory bowel disease: an update
Techniques in Coloproctology (2020)
-
MicroRNA 375 modulates hyperglycemia-induced enteric glial cell apoptosis and Diabetes-induced gastrointestinal dysfunction by targeting Pdk1 and repressing PI3K/Akt pathway
Scientific Reports (2018)
-
Clostridium difficile-related postinfectious IBS: a case of enteroglial microbiological stalking and/or the solution of a conundrum?
Cellular and Molecular Life Sciences (2018)
-
Enteric glial cells counteract Clostridium difficile Toxin B through a NADPH oxidase/ROS/JNK/caspase-3 axis, without involving mitochondrial pathways
Scientific Reports (2017)
-
HIV-1 Tat-induced diarrhea evokes an enteric glia-dependent neuroinflammatory response in the central nervous system
Scientific Reports (2017)