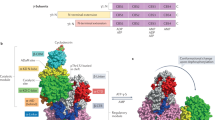
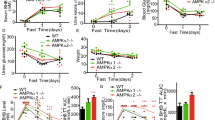
Abstract
5′AMP-activated protein kinase (AMPK) is recognized as an important intracellular energy sensor, shutting down energy-consuming processes and turning on energy-generating processes. Discovery of target proteins of AMPK has dramatically increased in the past 10 years. Historically, AMPK was first shown to regulate fatty acid and cholesterol synthesis, but is now hypothesized to take part in the regulation of energy/fuel balance not only at the cellular level but also at the level of the whole organism. In this brief review we will discuss some of the roles of AMPK in skeletal muscle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kahn BB, Alquier T, Carling D, Hardie DG . AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005; 1: 15–25.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–1174.
Fryer LGD, Parbu-Patel A, Carling D . The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 2002; 277: 25226–25232.
Wojtaszewski JFP, Birk JB, Frosig C, Holten M, Pilegaard H, Dela F . 5′AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. J Physiol 2005; 564: 563–573.
Hardie DG, Sakamoto K . AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 2006; 21: 48–60.
Birk JB, Wojtaszewski JFP . Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol 2006; 577 (Part 3): 1021–1032.
Chen Z, Heierhorst J, Mann RJ, Mitchelhill KI, Michell BJ, Witters LA et al. Expression of the AMP-activated protein kinase beta1 and beta2 subunits in skeletal muscle. FEBS Lett 1999; 460: 343–348.
Mahlapuu M, Johansson C, Lindgren K, Hjälm G, Barnes BR, Krook A et al. Expression profiling of the gamma-subunit isoforms of AMP-activated protein kinase suggests a major role for gamma3 in white skeletal muscle. Am J Physiol Endocrinol Metab 2004; 286: E194–E200.
Yu H, Fujii N, Hirshman MF, Pomerleau JM, Goodyear LJ . Cloning and characterization of mouse 5′-AMP-activated protein kinase gamma3 subunit. Am J Physiol Cell Physiol 2004; 286: C283–C292.
Cheung PCF, Salt IP, Davies SP, Hardie DG, Carling D . Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J 2000; 346: 659–669.
Sander MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D . Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 2007; 403: 139–148.
Riek U, Scholz R, Konarev P, Rufer A, Suter M, Nazabal A et al. Structural properties of AMP-activated protein kinase: Dimerization, molecular shape, and changes upon ligand binding. JBC 2008; 283: 18331–18343.
Witters LA, Kemp BE, Means AR . Chutes and Ladders: the search for protein kinases that act on AMPK. Trends Biochem Sci 2006; 31: 13–16.
Hardie DG, Carling D, Carlson M . The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 1998; 67: 821–855.
Thomson DM, Porter BB, Tall JH, Kim HJ, Barrow JR, Winder WW . Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am J Physiol Endocrinol Metab 2006; 292: E196–E202.
Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 2005; 24: 1810–1820.
Jørgensen SB, Richter EA, Wojtaszewski JFP . Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J Physiol 2006; 574: 17–31.
Wojtaszewski JFP, Mourtzakis M, Hillig T, Saltin B, Pilegaard H . Dissociation of AMPK activity and ACCbeta phosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun 2002; 298: 309–316.
Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D et al. Leptin stimulates fatty-acid oxidation by activation AMP-activated protein kinase. Nature 2002; 415: 339–343.
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8: 1288–1295.
Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X et al. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun 2004; 320: 449–454.
Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ . Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 1998; 47: 1369–1373.
Kristensen JM, Johnsen AB, Brik JB, Nielsen JN, Jensen BR, Hellsten Y et al. Absence of humoral mediated 5′AMP-activated protein kinase activation in human skeletal muscle and adipose tissue during exercise. J Physiol 2007; 585: 897–909.
Sandström ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H et al. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol 2006; 575: 251–262.
Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF . AS160 phosphorylation is associated with activation of alpha2beta2gamma1 but not alpha2beta2gamma3 AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 2006; 292: E715–E722.
Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 2006; 55: 2051–2058.
Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE et al. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 2006; 55: 2067–2076.
Norman B, Sollevi A, Kaijser L, Jansson E . ATP breakdown products in human skeletal muscle during prolonged exercise to exhaustion. Clin Physiol 1987; 7: 503–510.
Sahlin K, Broberg S, Ren JM . Formation of inosine monophosphate (IMP) in human skeletal muscle during incremental dynamic exercise. Acta Physiol Scand 1989; 136: 193–198.
Jørgensen SB, Wojtaszewski JFP, Viollet B, Andreelli F, Birk JB, Hellsten Y et al. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J 2005; 19: 1146–1148.
Klein DK, Pilegaard H, Treebak JT, Jensen TE, Viollet B, Schjerling P et al. Lack of AMPKα2 enchances pyruvate dehydrogenase activity during exercise. Am J physiol Endocrinol Metab 2007; 293: E1242–E1249.
Jørgensen SB, Viollet B, Andreelli F, Frøsig C, Birk JB, Schjerling P et al. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 2004; 279: 1070–1079.
Mu J, Brozinick Jr JT, Valladares O, Bucan M, Birnbaum MJ . A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 2001; 7: 1085–1094.
Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM et al. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem 2005; 280: 39033–39041.
Barnes BR, Glund S, Long YC, Hjalm G, Andersson L, Zierath JR . 5′-AMP-activated protein kinase regulates skeletal muscle glycogen content and ergogenics. FASEB J 2005; 19: 773–779.
Mu J, Barton ER, Birnbaum MJ . Selective suppression of AMP-activated protein kinase in skeletal muscle: update on ‘lazy mice’. Biochem Soc Trans 2003; 31: 236–241.
Fujii N, Seifert MM, Kane EM, Peter LE, Ho RC, Winstead S et al. Role of AMP-activated protein kinase in exercise capacity, whole body glucose homeostasis, and glucose transport in skeletal muscle -insight from analysis of a transgenic mouse model-. Diabetes Res Clin Prac 2007; 77S: S92–S98.
Pederson BA, Cope CR, Irimia JM, Schroeder JM, Thurberg BL, Depaoli-Roach AA et al. Mice with elevated muscle glycogen stores do not have improved exercise performance. Biochem Biophys Res Commun 2005; 331: 491–496.
Pederson BA, Cope CR, Schroeder JM, Smith MW, Irimia JM, Thurberg BL et al. Exercise capacity of mice genetically lacking muscle glycogen synthase: in mice, muscle glycogen is not essential for exercise. J Biol Chem 2005; 280: 17260–17265.
Camacho RC, Donahue EP, James FD, Berglund ED, Wasserman DH . Energy state of the liver during short-term and exhaustive exercise in C57BL/6J mice. Am J Physiol Endocrinol Metab 2006; 290: E405–E408.
Shoubridge EA, Challiss RA, Hayes DJ, Radda GK . Biochemical adaptation in the skeletal muscle of rats depleted of creatine with the substrate analogue beta-guanidinopropionic acid. Biochem J 1985; 232: 125–131.
Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO . Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 2000; 88: 2219–2226.
Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab 2001; 281: E1340–E1346.
Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA 2002; 99: 15983–15987.
Jørgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF et al. Role of alpha2-AMPK in basal, training- and AICAR-induced GLUT4, hexokinase II and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab 2006; 292: E331–E339.
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999; 98: 115–124.
Terada S, Tabata I . Effects of acute bouts of running and swimming exercise on PGC-1alpha protein expression in rat epitrochlearis and soleus muscle. Am J Physiol Endocrinol Metab 2004; 286: E208–E216.
Wojtaszewski JF, Nielsen JN, Richter EA . Invited review: effect of acute exercise on insulin signaling and action in humans. J Appl Physiol 2002; 93: 384–392.
Wojtaszewski JF, Hansen BF, Kiens B, Richter EA . Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes 1997; 46: 1775–1781.
Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB et al. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes 2002; 51: 2886–2894.
Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA . Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 2002; 282: E18–E23.
Watson RT, Pessin JE . Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem Sci 2006; 31: 215–222.
Mîinea CP, Sano H, Kane S, Sano E, Fukuda M, Peränen J et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J 2005; 391: 87–93.
Larance M, Ramm G, Stöckli J, van Dam EM, Winata S, Wasinger V et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem 2005; 280: 37803–37813.
Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ . AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 2006; 281: 31478–31485.
Acknowledgements
This review was supported by funds from the Commission of the European Communities (contract no. LSHM-CT-2004-005272 EXGENESIS and contract no. LSHM-CT-2004-512013 EUGENE2), the Danish Medical Research Council, the Novo Nordisk Foundation, the Danish Diabetes Association and the Copenhagen Muscle Research Centre. JFPW was supported by a Hallas Møller fellowship from the Novo Nordisk Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors state no conflict of interest.
Rights and permissions
About this article
Cite this article
Treebak, J., Wojtaszewski, J. Role of 5′AMP-activated protein kinase in skeletal muscle. Int J Obes 32 (Suppl 4), S13–S17 (2008). https://doi.org/10.1038/ijo.2008.117
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.117
Keywords
This article is cited by
-
Impaired insulin-induced site-specific phosphorylation of TBC1 domain family, member 4 (TBC1D4) in skeletal muscle of type 2 diabetes patients is restored by endurance exercise-training
Diabetologia (2011)
-
Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle
Diabetologia (2009)