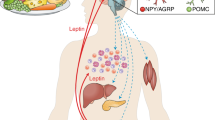
Abstract
Following the discovery of leptin in 1994, the scientific and clinical communities have held great hope that manipulation of the leptin axis may lead to the successful treatment of obesity. This hope is not yet dashed; however the role of the leptin axis is now being shown to be ever more complex than was first envisaged. It is now well established that leptin interacts with pathways in the central nervous system and through direct peripheral mechanisms. In this review, we consider the tissues in which leptin is synthesized and the mechanisms which mediate leptin synthesis, the structure of leptin and the knowledge gained from cloning leptin genes in aiding our understanding of the role of leptin in the periphery. The discoveries of expression of leptin receptor isotypes in a wide range of tissues in the body have encouraged investigation of leptin interactions in the periphery. Many of these interactions appear to be direct, however many are also centrally mediated. Discovery of the relative importance of the centrally mediated and peripheral interactions of leptin under different physiological states and the variations between species is beginning to show the complexity of the leptin axis. Leptin appears to have a range of roles as a growth factor in a range of cell types: as be a mediator of energy expenditure; as a permissive factor for puberty; as a signal of metabolic status and modulation between the foetus and the maternal metabolism; and perhaps importantly in all of these interactions, to also interact with other hormonal mediators and regulators of energy status and metabolism such as insulin, glucagon, the insulin-like growth factors, growth hormone and glucocorticoids. Surely, more interactions are yet to be discovered. Leptin appears to act as an endocrine and a paracrine factor and perhaps also as an autocrine factor. Although the complexity of the leptin axis indicates that it is unlikely that effective treatments for obesity will be simply derived, our improving knowledge and understanding of these complex interactions may point the way to the underlying physiology which predisposes some individuals to apparently unregulated weight gain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jebb SA . Obesity: from molecules to man Proc Nutr Soc 1999 58: 1–14.
Vanltallie TB . Worldwide epidemiology of obesity PharmacoEconom 1994 5 (Supp 1): 1–7.
Kuczmarski RJ, Fiegal KM, Campbell SM, Johnson CL . Increasing prevalence of overweight among US adults JAMA 1994 272: 205–211.
Brown W, Dobson A, Mishra G . What is a healthy range for middle aged women? Int J Obes Relat Metab Disord 1998 22: 520–528.
Strader CD, Hwa JJ, Van Heek M, Parker EM . Novel molecular targets for the treatment of obesity Drug Discov Today 1998 3: 250–256.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM . Positional cloning of the mouse obese gene and its human homologue Nature 1994 372: 425–432.
Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P . Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks Science 1995 269: 546–549.
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Robinowitz D, Lollone RL, Burley SK, Friedman JM . Weight-reducing effects of the plasma protein encoded by the obese gene Science 1995 269: 543–546.
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F . Effects of the obese gene product on body weight regulation in ob/ob mice Science 1995 269: 540–543.
Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards JG, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Woolf EA, Monroe CA, Tepper RI . Identification and expression cloning of a leptin receptor, OB-R Cell 1995 83: 1263–1271.
Lee G-H, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM . Abnormal splicing of the leptin receptor in mice Nature 1996 379: 632–635.
Bray GA, York DA . Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis Physiol Rev 1979 59: 719–809.
Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG . Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta Proc Natl Acad Sci USA 1997 94: 11073–11078.
Hoggard N, Mercer JG, Rayner DV, Moar K, Trayhurn P, Williams LM . Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridisation Biochem Biophys Res Commun 1997 232: 383–387.
Spicer LJ, Francisco CC . The adipose obese gene product, leptin: evidence of direct inhibitory role in ovarian function Endocrinology 1997 138: 3374–3379.
Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau J-P, Bortoluzzi M-N, Moizo L, Lehy T, Buerre-Millo M, Le Marchand-Brustel Y, Lewin MJM . The stomach is a source of leptin Nature 1998 394: 790–793.
Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L . A nutrient-sensing pathway regulates leptin gene expression in muscle and fat Nature 1998 393: 684–688.
Considine RV, Caro JF . Leptin and the regulation of body weight Int J Biochem Cell Biol 1997 11: 1255–1272.
Moinat M, Deng C, Muzzin P, Assimocopoulos-Jeannet F, Seydoux J, Dulloo AG, Giacobino JP . Modulation of obese gene expression in rat brown and white adipose tissues FEBS Lett 1995 373: 131–134.
Tsuro Y, Sato I, Iida M, Murakami T, Ishimura K, Shima K . Immunohistochemical detection of the ob gene product (leptin) in rat white and brown adipocytes Horm Metab Res 1996 28: 753–755.
Klingenspor M, Dickopp A, Heldmaier G, Klaus S . Short photoperiod reduces leptin gene expression in white and brown adipose tissue of Djungarian hamsters FEBS Lett 1996 399: 290–294.
Deng C, Moinat M, Curtis L, Nadakal A, Preinter F, Boss O, Assimacopoulos-Jeannet F, Sedoux J, Giacobino J-P . Effects of b-adrenoceptor subtype stimulation on obese gene mRNA and on leptin secretion in mouse brown adipocytes differentiated in culture Endocrinology 1997 138: 548–552.
Dessolin S, Schalling M, Champigny O, Lonnqvist F, Alhaud G, Dani C, Ricquier D . Leptin gene is expressed in rat brown adipose tissue at birth FASEB J 1997 11: 382–387.
Cinti S, Frederich RC, Zingaretti MC, De Matteis R, Flier JS, Lowell BB . Immunohistochemical localisation of leptin and uncoupling protein in white and brown adipose tissue Endocrinology 1997 138: 797–804.
Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW, Cusin I, Rohner-Jeanrenaud C, Burger AG, Zapf J, Meier CA . Direct effects of leptin on brown and white tissues J Clin Invest 1997 100: 2858–2864.
Kutoh E, Boss O, Levasseur F, Giacobino J-P . Quantification of the full length leptin receptor (OB-Rb) in human brown and white adiposse tissue Life Sci 1998 62: 445–451.
Giacobino JP . Role of β3-adrenoceptor in the control of leptin expression Horm Metab Res 1996 28: 633–637.
Trayhurn P, Duncan JS, Rayner DV . Acute cold-induced suppression of ob (obese) gene expression in white adipose tissue of mice; mediation by the sympathetic system Biochem J 1995 311: 729–733.
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM . Leptin levels in human and rodents measurement of plasma leptin and ob RNA in obese and weight-reduced subjects Nat Med 1995 1: 1155–1161.
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF . Serum immunoreactive-leptin concentrations in normal-weight and obese humans New J Engl Med 1996 334: 292–295.
Hube F, Lietz U, Igel M, Jensen PB, Tornqvist H, Joost H-G, Hauner H . Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans Horm Metab Res 1996 28: 690–693.
Masuzaki H, Ogawa Y, Isse N, Satoh N, Okazaki T, Shigemoto M, Mori K, Tamura N, Hosoda K, Yoshimasa Y, Jingami H, Kawada T, Nakao K . Human obese gene expression: adipocyte-specific expression and regional differences in the adipose tissue Diabetes 1995 44: 855–858.
Montague CT, Prins JB, Sanders L, Digby JE, O'Rahilly S . Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution Diabetes 1997 46: 342–347.
Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, Arner P . Leptin secretion from subcutaneous and visceral adipose tissue in women Diabetes 1998 47: 913–917.
Lonnqvist F, Arner P, Nordfors L, Schalling M . Overexpression of the obese (ob) gene in adipose tissue of human obese subjects Nat Med 1995 1: 950–953.
Rayner DV, Dalgliesh GD, Duncan JS, Hardie LJ, Hoggard N, Trayhurn P . Postnatal development of the ob gene system: elevated leptin levels in suckling fa/fa rats Am J Physiol 1997 42: R446–R450.
Machinal F, Dieudonne M-N, Leneveu M-C, Pecquery R, Giudicelli Y . In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones Endocrinology 1999 140: 1567–1574.
Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL . Effects of gender, body composition, and menopause on plasma concentrations of leptin J Clin Endocrinol Metab 1996 81: 3424–3427.
Kennedy A, Gettys TW, Watson, Wallace P, Ganaway E, Pan Q, Garvey WT . The metabolic significance of leptin in humans; gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure J Clin Endocrinol Metab 1997 82: 1293–1300.
Saad MF, Damani S, Gingerich RL . Sexual dimorphism in plasma leptin concentration J Clin Endocrinol Metab 1997 82: 579–584.
Hassink WG, de Lancey E, Sheslow DV, Smith-Kirwin SM, O'Connor, Considine RVOI, Dostal K, Spear ML, Leef K, Ash M, Spitzer AR, Funange VL . Placental leptin: an important new growth factor in intrauterine and neonatal development? Pediatrics 1997 100: 1–6.
Masuzaki H, Ogava Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K . Nonadipose tissue production of leptin; leptin as a novel placenta-derived hormone in humans Nat Med 1997 3: 1029–1033.
Bennett PA, Lindell K, Wilson C, Carlsson LMS, Carlsson B, Robinson CAF . Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrus cycle Neuroendocrinology 1999 69: 417–423.
Senaris R, Garcia-Caballero T, Casabiell X, Gallego R, Castro R, Considine RV, Dieguez C, Casanueva FF . Synthesis of leptin inhuman placenta Endocrinology 1997 138: 4501–4504.
Bodner J, Ebenbichler CF, Wolf HJ, Muller-Holzner E, Stanzi U, Gander R, Huter O, Patsch JR . Leptin receptor in human term placenta: in situ hybridisation and immunohistochemical localisation Placenta 1999 20: 677–682.
Hoggard N, Hunter L, Trayhurn P, Williams LM, Mercer KG . Leptin and reproduction Proc Nutr Soc 1998 57: 421–427.
Lostao MP, Urdaneta E, Martinez-Anso E, Barber A, Martinez JA . Presence of leptin receptors in rat small intestine and leptin effect on sugar absorption FEBS Lett 1998 423: 302–306.
Morash B, Li A, Murphy PR, Wilkinson M, Ur E . Leptin gene expression in the brain and pituitary gland Endocrinology 1999 140: 5995–5998.
Jin L, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV . Leptin and leptin receptors expression in rat and mouse pituitary cells Endocrinology 2000 141: 333–339.
Smith-Kirwin SM, O'Connor DM, De Fohnston J, DeLancey ED, Hassink SG, Funanage VL . Leptin expression in human mammary epithelial cells and breast milk J Clin Endocrinol Metab 1998 83: 1810–1813.
Laharrague P, Larroy D, Fontanilles A-M, Truel N, Campfield A, Tenenbaum R, Galitzky J, Corberand JX, Penicaud L, Casteilla L . High expression of leptin by human bone marrow adipocytes in primary culture FASEB J 1998 12: 747–752.
Chen S-C, Kochan JP, Campfield A, Burn P, Smeyne RJ . Splice variants of the ob receptor gene are differentially expressed in brain and peripheral tissues of mice J Recept Signal Transm R 1999 19: 245–266.
Soukas A, Cohen P, Friedman JM . Gene expression profile induced by leptin in white adipose tissue and liver Nat Gen 1999 23: 75.
Taouis M, Chen J-W, Daviaud C, Dupont J, Derouet M, Simon J . Cloning the chicken leptin gene Gene 1998 208: 239–242.
Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS . Expression of ob mRNA and its encoded protein in rodents: impact of nutrition and obesity J Clin Invest 1995 96: 1659–1663.
Fruhbeck G, Aguardo M, Martinez JA . In vitro lipolytic effect of leptin on mouse adipocytes; evidence for a possible autocrine-paracrine role of leptin Biochem Biophys Res Commun 1997 240: 590–594.
Maffei M, Fei H, Lee G-H, Dani C, Leroy P, Zhang Y, Proenca R, Nagrel R, Ailhaud G, Friedman J . Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus Proc Natl Acad Sci USA 1995 92: 6957–6960.
Masuzaki H, Ogawa Y, Isse N, Satoh N, Okazaki T, Shigemoto M, Mori K, Tamura N, Hosoda K, Yoshimasa Y, Jingami H, Kawada T, Nakao K . Human obese gene expression: Adipocyte-specific expression and regional differences in the adipose tissue Diabetes 1995 44: 855–858.
Arner P . Impact of exercise on adipose tissue metabolism in humans Int J Obes Relat Metab Disord 1995 19: S18–S21.
Landt M, Lawson GM, Helgeson JM, Davila-Roman VG, Laderson JH, Jaffe AS, Hickner RC . Prolonged exercise decreases serum leptin concentrations Metabolism 1997 46: 1109–1112.
Weimann E, Blum WF, Witzel C, Schwidergall S, Bohles HJ . Hypoleptinemia in female and male elite gymnasts Eur J Clin Invest 1999 29: 853–860.
Zheng D, Wooter MH, Zhou Q, Dahm GL . The effect of exercise on ob gene expression Biochem Biophys Res Commun 1996 225: 747–750.
Kohrt W, Landt M, Birge SJJ . Serum leptin levels are reduced in response to exercise training, but not hormone replacement therapy, in older women J Clin Endocrinol Metab 1996 81: 3980–3985.
Racette SB, Coppack SW, Landt M, Klein S . Leptin production during moderate-intensity aerobic exercise J Clin Endocrinol Metab 1997 82: 2275–2277.
Hickey MS, Considine RV, Israel RG, Mahar TL, McCammon MR, Tyndall GL, Houmard JA, Caro JF . Leptin is related to body fat content in male distance runners Am J Physiol 1996 271: E938–E940.
Perusse L, Collier G, Gagnon J, Leon A, Rao DC, Skinner JS, Wilmore JH, Nadeau A, Zimmet PZ, Bouchard C . Acute and chronic effects of exercise on leptin levels in humans J Appl Physiol 1997 83: 5–10.
Hickey MS, Houmard JA, Considine RV, Tyndall GL, Midgette JB, Gavigan KE, Weidner ML, McCammon MR, Israel RG, Caro JF . Gender dependent effects of exercise training on serum leptin levels in humans Am J Physiol 1997 272: E562–E566.
Pasman WJ, Westerterp-Plantenga MS, Saris WHM . The effect of exercise training on leptin levels in obese males Am J Physiol 1998 274: E280–E286.
Fruhbeck G, Jebb SA, Prentice AM . Leptin; physiology and pathophysiology Clin Physiol 1998 18: 399–419.
Hardie LJ, Rayner DV, Holmes S, Trayhurn P . Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but Zucker (fa/fa) rats as measured by ELISA Biochem Biophys Res Commun 1996 223: 660–665.
MacDougald OA, Hwang CS, Fan H, Lane MD . Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes Proc Natl Acad Sci USA 1995 92: 9034–9037.
Peino R, Pineiro V, Gualillo O, Menendez C, Brenlla J, Casabiell X, Dieguez C, Casanueva FF . Cold exposure inhibits leptin secretion in vitro by a direct and non-specific action on adipose tissue Eur J Endocrinol 2000 142: 195–199.
Trayhurn P, Duncan JS, Rayner DV, Hardie LJ . Rapid inhibition of ob gene expression and circulating leptin levels in lean mice by the b3-adrenoceptor agonists BRL 35135A and ZD2079 Biochem Biophys Res Commun 1996 228: 605–610.
Hardie LJ, Guilhot N, Trayhurn P . Regulation of leptin production in cultured mature white adipocytes Horm Metab Res 1996 28: 685–689.
Leininger MT, Portocarrero CP, Schinckel AP, Spurlock ME, Bidwell CA, Nielsen JN, Houseknecht KL . Physiological response to acute endotoxemia in swine: effect of genotype on energy metabolites and leptin Domest Anim Endocrinol 2000 18: 71–82.
Boden G, Chen X, Mazzoli M, Ryan I . Effect of fasting on serum leptin in normal human subjects J Clin Endocrinol Metab 1996 81: 2419–3423.
Trayhurn P, Thomas MEA, Duncan JS, Rayner DV . Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (ob/ob) mice FEBS Lett 1995 368: 488–490.
Champigny O, Ricquier D . Effects of fasting and refeeding on the level of uncoupling protein mRNA in rat brown adipose tissue; evidence for diet-induced and cold-induced responses J Nutr 1990 120: 1730–1736.
Scarpace PJ, Matheny M . Leptin induction of UCP1 gene expression is dependent on sympathetic innervation Am J Physiol 1998 275: E259–E264.
Sivitz WI, Fink BD, Donohoue PA . Fasting and leptin modulate adipose and muscle uncoupling protein: divergent effects between messenger ribonucleic acid and protein expression Endocrinology 1999 140: 1511–1519.
Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, Ma Z, Vignati L, Bowsher R, Herzog D, Klibanski A . Serum leptin levels in women with anorexia nervosa J Clin Endocrinol Metab 1996 81: 3861–3863.
Deuschle M, Blum WF, Englaro P, Schweiger U, Weber B, Pflaum C-D, Heuser I . Plasma leptin in depressed patients and healthy controls Horm Metab Res 1996 28: 714–717.
Haluzik M, Kabrt J, Nedvidkova J, Svoboda J, Kotrlikova E, Papezova H . Relationship of serum leptin levels and selected nutritional parameters in patients with protein-caloric malnutrition Nutrition 1999 15: 829–833.
Mantzoros CS, Flier JS, Lesem MD, Brewerton TD, Jimerson DC . Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain J Clin Endocrinol Metab 1997 82: 1845–1851.
Stumvoll M, Fritsche A, Tschritter O, Lehmann R, Wahl HR, Renn W, Haring H . Leptin levels in humans are acutely suppressed by isoproterenol despite acipimox-induced inhibition of lipolysis, but not by free fatty acids Metabolism 2000 49: 335–339.
Mantzoros CS, Qu D, Frederich RC, Susulic VS, Lowell BB, Maratos-Flier E, Flier JS . Activation of β3 adrenergic receptors suppresses leptin expression and mediates a leptin independent inhibition of food intake in mice Diabetes 1996 45: 909–914.
Li H, Matheny M, Scarpace PJ . b3-adrenergic-mediated suppression of leptin gene expression in rats Am J Physiol 1997 272: E1031–E1036.
Donahoo WT, Jensen DR, Yost TJ, Eckel RH . Isoproterenol and somatostatin decrease plasma leptin in humans; a novel mechanism regulating leptin secretion J Clin Endocrinol Metab 1997 82: 4139–4143.
Enocksson S, Shimizu M, Lonnqvist F, Nordenstrom J, Arner P . Demonstration of an in vivo functional β3-adrenoceptor in man J Clin Invest 1995 95: 2239–2245.
Trayhurn PHN, Mercer JG, Rayner DV . Leptin: fundamental aspects Int J Obes Relat Metab Disord 1999 23 (Suppl 1): 22–28.
Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS . Role of leptin in fat regulation Nature 1996 380: 677.
Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI . Receptor-mediated regional sympathetic nerve activation by leptin J Clin Invest 1997 100: 270–278.
Dunbar JC, Hu Y, Lu H . Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats Diabetes 1997 46: 2040–2043.
He Y, Chen H, Quon MJ, Reitman M . The mouse obese gene: genomic organisation, promoter activity, and activation by CCAAT/enhancer-binding protein a J Biol Chem 1995 270: 28887–28891.
Miller SG, De Vos P, Guerre-Millo M, Wong K, Hermann T, Staels B, Briggs MR, Auwerx J . The adipocyte specific transcription factor C/EBPa modulates human ob gene expression Proc Natl Acad Sci USA 1996 93: 5507–5511.
De Vos P, Lefebve AM, Miller SG, Guerre-Millo M, Wong K, Saladin R, Hamann LG, Steals B, Briggs MR, Auwerx J . Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor gamma J Clin Invest 1996 98: 1004–1009.
Kallen CB, Lazar MA . Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes Proc Natl Acad Sci USA 1996 93: 5793–5796.
Tartaglia L . The leptin receptor J Biol Chem 1997 272: 6093–6096.
Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC . Defective STAT signaling by the leptin receptor in diabetic mice Proc Natl Acad Sci USA 1996 93: 6231–6235.
Dvan Heek M, Mullins DE, Wirth MA, Graziano MP, Fawzi AB, Compton DS, France CF, Hoos LM, Casale RL, Sybertz EJ, Strader CD, Davis HRJ . The relationship of tissue localization, distribution and turnover to feeding after intraperitoneal 125I-leptin administration to ob/ob and db/db mice Horm Metab Res 1996 28: 653–658.
De Matteis R, Dashtipour K, Ognibene A, Cinti S . Localisation of leptin receptor splice variants in mouse peripheral tissues by immunohistochemistry Proc Nutr Soc 1998 57: 441–448.
Kieffer TJ, Heller RS, Habaner JF . Leptin receptors expressed on pancreatic b-cells Biochem Biophys Res Commun 1996 224: 522–527.
Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P . Localisation of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridisation FEBS Lett 1996 387: 113–116.
Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM . Anatomical localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues Proc Natl Acad Sci USA 1997 94: 7001–7005.
Stephens TW, Basinski M, Bristow PK, Blue-Valleskey JM, Burgett G, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, MacKeller W, Rosteck PR, Schoner B Jr, Smith D, Tinsley FC, Zhang XY, Heiman M . The role of neuropeptide Y in the antiobesity action of the obese gene product Nature 1995 377: 530–532.
Heaney ML, Golde DW . Soluble hormone receptors Blood 1993 82: 1945–1948.
Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail D, Platika D, Snodrass . Novel B219/OB receptor isoforms; possible role of leptin in hematopoieses and reproduction Nat Med 1996 2: 585–589.
Bazan JF . Structural design and molecular evolution of a cytokine receptor superfamily Proc Natl Acad Sci USA 1990 87: 6934–6938.
Miyazaki T, Maruyama M, Yamada G, Hatekeyama M, Taniguchi T . The integrity of the conserved WS motif common to IL-2 and other receptors is essential for ligand binding and signal transduction EMBO J 1991 10: 3191–3197.
Ihle JM . Cytokine receptor signalling Nature 1995 377: 591–594.
Fong TM, Huang R-RC, Tota MR, Mao C, Smith T, Varnerin J, Karpitskiy VV, Krause JE, Van der Ploeg LHT . Localization of leptin binding domain in the leptin receptor Mol Pharmac 1998 53: 234–240.
Chen H, Charlat O, Tartaglia LA, Woolf EA, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP . Evidence that the leptin diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice Cell 1996 84: 491–495.
Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai C-F, Tartaglia LA . The full-length leptin receptor has signalling capabilities of interleukin 6-type cytokine receptors Proc Natl Acad Sci USA 1996 93: 8374–8378.
Yamashita T, Murakami T, Iida M, Kuwajima M, Shima K . Leptin receptor of Zucker fatty rat performs reduced signal transduction Diabetes 1997 46: 1077–1080.
Devos R, Guisez Y, Van der Heyden J, White DW, Kalai M, Fountoulakis M, Plaetinck G . Ligand-independent dimerisation of the extracellular domain of the leptin receptor and determination of the stoichiometry of leptin binding J Biol Chem 1997 272: 18304–18310.
White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA . Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization J Biol Chem 1997 272: 4065–4071.
White DW, Tartaglia LA . Evidence for ligand-independent homo-oligomerisation of leptin receptor (OB-R) isoforms: a proposed mechanism permitting productive long-form signalling in the presence of excess short-form expression J Cell Biochem 1999 73: 278–288.
Bjorbaek CUS, Da Silva B, Flier JS . Divergent signaling capacities of the long and short isoforms of the leptin receptor J Biol Chem 1997 272: 32686–32695.
Houseknecht KL, Portocarrero CP . Leptin and its receptors of whole-body energy homeostasis Domest Anim Endocrinol 1998 15: 457–475.
Ghilardi N, Skoda RC . The leptin receptor activates Janus Kinase 2 and signals for proliferation in a factor-dependent cell line Mol Endocrinol 1997 11: 393–399.
Lebrun J-J, Ali S, Ullrich A, Kelly PA . Proline-rich sequence-mediated Jak2 association to the prolactin receptor is required but not sufficient for signal transduction J Biol Chem 1995 270: 10664–10670.
Joneja B, Wojchowski DM . Mitogenic signaling and inhibition of apoptosis via the erythropoietin receptor Box-1 domain J Biol Chem 1997 272: 11176–11184.
Vaisse C, Halaas JL, Horvath CM, Darnell JE, Stoffel M, Friedman JM . Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice Nat Genet 1996 14: 95–97.
White DW, Wang DW, Chua SCJ, Morgenstern JP, Leibel RL, Baumann H, Tartaglia LA . Constitutive and impaired signaling of leptin receptors containing the Gln-Pro extracellular domain fatty mutation Proc Natl Acad Sci USA 1997 94: 10657–10662.
Emilsson V, Arch JRS, de Groot RP, Lister CA, Cawthorne MA . Leptin treatment increases suppressors of cytokine signalling in central and peripheral tissues FEBS Lett 1999 455: 170–174.
Rosenblum C, Tota M, Cilly D, Smith T, Cullum R, Qureshi S, Hess JF, Philips MS, Hey P, Vongs A, Fong TM, Xu L, Chen HY, Smith RG, Schindler, Van Der Ploeg LHT . Functional STAT1 and 3 signalling by the leptin receptor (OB-R); reduced expression of he rat fatty leptin receptor in transfected cells Endocrinology 1996 137: 5178–5181.
Stromberg H, Svensson SPS, Hermanson O . Distribution of the transcription factor Signal Transducer and Activator of Transcription 3 in the rat central nervous system and dorsal root ganglia Brain Res 2000 853: 105–114.
Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ . A family of cytokine-inducible inhibitors of signalling Nature 1997 387: 917–921.
Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A . A new protein containing SH2 domain that inhibits JAK kinasas Nature 1997 387: 921–924.
Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T . Structure and function of a new STAT-induced STAT inhibitor Nature 1997 387: 924–929.
Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS . Identification of SOCS-3 as a potential mediator of central leptin resistance Mol Cell 1998 1: 619–625.
Bjorbaek C, El-haschimi K, Frantz JD, Flier JS . The role of SOCS-3 in leptin signaling and leptin resistance J Biol Chem 1999 274: 30059–30065.
Banks WA, Clever CM, Farrell CL . Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice Am J Physiol 2000 278: E1158–E1165.
Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM . Leptin enters the brain by a saturable system independent of insulin Peptides 1996 17: 305–311.
Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberg P . Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor Peptides 1999 20: 1449–1453.
Murakami T, Yamashita T, Ilida M, Kuwajima M, Shima K . A short for of the leptin receptor performs signal transduction Biochem Biophys Res Commun 1997 231: 26–29.
Wang M, Zhou Y, Newgard C, Unger R . A novel leptin receptor isoform in rat FEBS Lett 1996 392: 87.
Chua SCJ, Koutras IK, Han L, Liu S-M, Kay J, Young SJ, Chung WK, Leibel RL . Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts Genomics 1997 45: 264–270.
Lewandowski K, Horn R, O'Callaghan CJ, Dunlop D, Medley G, O'Hare P, Brabant G . Free leptin, bound leptin and soluble leptin receptor in normal and diabetic pregnancies J Clin Endocrinol Metab 1999 84: 300–306.
Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B . A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction Nature 1998 392: 398–401.
Lahlou N, Clement K, Carel JC, Vaisse C, Lotton C, Le Bihan Y, Basdevant A, Lebouc Y, Froguel P, Roger M, Guy-Grand B . Soluble leptin receptor in serum of subjects with complete resistance to leptin: relation to fat mass Diabetes 2000 49: 1347–1352.
Lollmann B, Gruninger S, Stricker-Krongrad A, Chiesi M . Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and e in different mouse tissues Biochem Biophys Res Commun 1997 238: 648–652.
Takekoshi K, Motooka M, Isobe K, Nomura F, Manmoku T, Ishii K, Nakai T . Leptin directly stimulates catecholamine secretion and synthesis in cultured porcine adrenal medullary chromaffin cells Biochem Biophys Res Commun 1999 261: 426–431.
Serradeil-Le Gal C, Raufaste D, Brossard G, Pouzet B, Marty E, Maffrand J-P, Le Fur G . Characterization and localization of leptin receptors in the rat kidney FEBS Lett 1997 404: 185–191.
Malik KF, Young WS III . Localisation of binding sites in the central nervous system for leptin (Ob protein) in normal, obese (ob/ob), and diabetic (db/db) C57BL/6J mice Endocrinology 1996 137: 1497–1500.
Golden PL, Maccagnan TJ, Pardridge WM . Human blood-brain barrier leptin receptor J Clin Invest 1997 99: 14–18.
Corp ES, Conze DB, Smith F, Campfield LA . Regional localisation of specific [125I] leptin binding sites in rat forebrain Brain Res 1998 789: 40–47.
Wang Y, Kuropatwinski KK, White DW, Hawley TS, Hawley RG, Tartaglia LA, Baumann H . Leptin receptor action in hepatic cells J Biol Chem 1997 272: 16216–16223.
Uotani S, Bjorbak, Tornoe J, Flier JS . Functional properties of leptin receptor isoforms; internationalisation and degradation of leptin and ligand-induced receptor downregulation Diabetes 1999 48: 279–286.
Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG . Identification of targets of leptin action in rat hypothalamus J Clin Invest 1996 98: 1101–1106.
Mercer JG, Moar KM, Hoggard N . Localisation of leptin receptor (Ob-R) messenger ribonucleic acid in the rodent hindbrain Endocrinology 1998 139: 29–34.
Cao G-Y, Considine RV, Lynn RB . Leptin receptors in the adrenal medulla of the rat Am J Physiol 1997 273: E448–E452.
Tsuchiya T, Shimizu H, Horie T, Mori M . Expression of leptin receptors in lung: leptin as a growth factor Eur J Pharmac 1999 365: 273–279.
Dal Farra C, Zsurger N, Vincent JP, Cupo A . Binding of a pure 125I-monoiodoleptin analog to mouse tissues: a developmental study Peptides 2000 21: 577–587.
Hill RA, Margetic S, Pegg G, Gazzola C . Leptin: its pharmokinetics and tissue distribution Int J Obes Relat Metab Disord 1998 22: 765–770.
Zeng J, Patterson B, Klein S, Martin DR, Dagogo-Jack S, Kohrt WM, Miller SB, Landt M . Whole body leptin kinetics and renal metabolism in vivo Am J Physiol 1997 273: E1102–E1106.
Gray RS, Cowan P, di Mario U, Elton RA, Clarke BF, Duncan LJP . Influence of insulin antibodies on pharmacokinetics and bioavailability of recombinant human and highly purified beef insulins in insulin dependent diabetics Br Med J 1985 290: 1687–1691.
Hill RA, Flick-Smith FC, Dye S, Pell JM . Actions of an IGF-1-enhancing antibody on IGF-1 pharmacokinetics and tissue distribution: increased IGF-1 bioavailability J Endocrinol 1997 152: 123–130.
Bastian SEP, Walton PE, Wallace JC, Ballard FJ . Plasma clearance and tissue distribution of labelled insulin-like growth factor-1 (IGF-1) and an analogue LR3IGF-1 in pregnant rats J Endocrinol 1993 138: 327–336.
Klein S, Coppack SW, Mohamed-Ali V, Landt M . Adipose tissue production and plasma leptin kinetics in humans Diabetes 1996 45: 984–987.
Ahren B, Baldwin RM, Have lPJ . Pharmokinetics of human leptin in mice and rhesus monkeys Int J Obes Relat Metab Disord 2000 24: 1579–1585.
Houseknecht KL, Mantzoros CS, Kuliawat R, Hadro E, Flier JS, Kahn BB . Evidence for leptin binding proteins in serum of rodents and humans; modulation with obesity Diabetes 1996 45: 1638–1643.
Sinha MK, Opentanova I, Ohannesian JP . Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting J Clin Invest 1996 98: 1277–1282.
Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, Nicola NA, Alexander WS, Hilton DJ . Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells Proc Natl Acad Sci USA 1996 93: 14564–14568.
Gainsford T, Alexander WS . A role for leptin in hemopoieses? Mol Biotech 1999 11: 149–158.
Umemoto Y, Tsuji K, Yang FC, Ebihara Y, Kaneko A, Furukawa S, Nakahata T . Leptin stimulates the proliferation of murine myoletic and primitive hematopoietic progenitor cells Blood 1997 90: 3438–3443.
Nakata M, Yada T, Soejima N, Maruyama I . Leptin promotes aggregation of human platelets via the long form of its receptor Diabetes 1999 48: 426–429.
Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapatropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverinin PJ, Flores-Riveros JR . Biological action of leptin as an angiogenic factor Science 1998 281: 1583–1585.
Bouloumie A, Drexler HCA, Lafontan M, Busse R . Leptin, the product of ob gene, promotes angiogenesis Circul Res 1998 83: 1059–1066.
Kang S-K, Kwon HM, Hong BK, Kim D, Kim IJ, Choi EY, Jang Y, Kim H-S, Kim MS, Kwon HC . Expression of leptin receptor (OB-R) in human atherosclerotic lesions: potential role in intimal neovascularisation Yonsei Med J 2000 41: 68–75.
Ring BD, Scully S, Davis CR, Baker MB, Cullen MJ, Pelleymounter MA, Danilenko DM . Systemically and topically administered leptin both accelerate wound healing in diabetic ob/ob mice Endocrinology 2000 141: 446–449.
Boado RJ, Golden PL, Levin N, Pardridge WM . Up-regulation of blood-brain barrier short-form leptin receptor gene products in rats fed a high fat diet J Neurochem 1998 71: 1761–1764.
Margetic S, Qui XY, D'Occio MJ, Gazzola C, Hill RA . Effect of mouse leptin on angiogenesis 15th Australasian Biotechnology Conference 2000 p 79.
Chehab FF, Mounzih K, Lu R, Lim ME . Early onset of reproductive function in normal female mice treated with leptin Science 1997 275: 88–90.
Ashworth CJ, Hoggard N, Thomas L, Mercer JG, Wallace JM, Lea RG . Placental leptin Rev Reprod 2000 5: 18–24.
Chehab FF, Lim ME, Lu RH . Correction of the sterility defect in homozygous obese female mice is treatment with the human recombinant leptin Nat Genet 1996 12: 318–320.
Spicer LJ, Francisco CC . Adipose obese gene product, leptin inhibits bovine ovarian thecal cell steriodogenesis Biol Reprod 1998 58: 207–212.
Karlsson C, Lindell K, Svenssen E, Bergh C, Lind P, Billing H, Carlsson LMS, Carlsson B . Expression of functional leptin receptors in the human ovary J Clin Endocrinol Metab 1997 82: 4144–4148.
Lindheim SR, Sauer MV, Carmina E, Chang PL, Zimmerman R, Lobo RA . Circulating leptin levels during ovulation induction: relation to adiposity and ovarian morphology Fertil Steril 2000 73: 493–498.
Barash I, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA . Leptin is metabolic signal to the reproductive system Endocrinology 1996 137: 3144–3147.
Mounzih K, Lu R, Chehab FF . Leptin treatment rescues the sterility of genetically obese ob/ob males Endocrinology 1997 138: 1190–1193.
Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, Mori M . Oestrogen increases in vivo leptin production in rats and human subjects J Endocrinol 1997 154: 285–292.
Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, Rascher W, Teller W, Tornquist H, Hauner H . Insulin and cortisol promote leptin production in cultured human cells Diabetes 1996 45: 1435–1438.
Jockenhovel F, Blum WF, Vogel E, Englaro P, Muler-Wieland D, Reinwein D, Rascher W, Krone W . Testosterone substitution normalises elevated serum leptin levels in hypogonadal men J Clin Endocrinol Metab 1997 82: 2510–2513.
Henson MC, Castracane VD . Leptin in pregnancy Biol Reprod 2000 63: 1219–1228.
Butte NF, Hopkinson JM, Nicolson MA . Leptin in human reproduction: serum leptin levels in pregnant and lactating women J Clin Endocrinol Metab 1997 82: 585–589.
Helland IB, Reseland JE, Saugstad OD, Drevon CA . Leptin levels in pregnant women and newborn infants: gender differences and reduction during the neonatal period Pediatrics 1998 101: 12.
Amico JA, Thomas A, Crowley RS, Burmeister LA . Concentrations of leptin in the serum of pregnant, lactating, and cycling rats and of leptin messenger ribonucleic acid in rat placental tissue Life Sci 1998 63: 1387–1395.
Terada Y, Yamakawa K, Sugaya A, Toyoda N . Serum leptin levels do not rise during pregnancy in age-matched rats Biochem Biophys Res Commun 1998 235: 841–844.
Henson MC, Swan KF, O'Neil JS . Expression of placental leptin and leptin receptor transcripts in early pregnancy and at term Obstet Gynecol 1998 92: 1020–1028.
Gavrilova O, Barr V, Marcus-Samuels B, Reitman M . Hyperleptinaemia of pregnancy associated with the appearance of a circulating form of the leptin receptor J Biol Chem 1997 272: 30546–30551.
Barb CR . The brain–pituitary–adipocyte axis: role of leptin in modulating neuroendocrine function J Anim Sci 1999 77: 1249–1257.
Holness MJ, Munns MJ, Sugden MC . Current concepts concerning the role of leptin in reproductive function Mol Cell Endocrinol 1999 157: 11–20.
Mounzih K, Qui J, Ewart-Toland A, Chehab FF . Leptin is not necessary for gestation and parturition but regulates maternal nutrition via a leptin resistance state Endocrinology 1998 139: 5259–5262.
Linnemann K, Malek A, Sager R, Blum WF, Schneider H, Fusch C . Leptin production and release in the dually in vitro perfused human placenta J Clin Endocrinol Metab 2000 85: 4298–4301.
Mukherjea R, Castonguay TW, Douglass LW, Moser-Veillon P . Elevated leptin concentrations in pregnancy and lactation: possible role as a modulator of substrate utilization Life Sci 1999 65: 1183–1193.
Schubring C, Kiess W, Englaro P, Rascher W, Dotsch J, Hanitsch S, Attanasio A, Blum WF . Levels of leptin in maternal serum, amniotic fluid, and arterial and venous cord blood: relation to neonatal and placental weight J Clin Endocrinol Metab 1997 82: 1480–1483.
Harigaya ANK, Nako Y, Morikawa A . Relationship between concentration of serum leptin and foetal growth J Clin Endocrinol Metab 1997 82: 3281–3284.
Matsuda J, Yokota I, Iida M, Murakami T, Naito E, Ito M, Shima K, Kuroda Y . Serum leptin concentration in cord blood: relationship to birth weight and gender J Clin Endocrinol Metab 1997 82: 1642–1644.
Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM . Role of leptin in hypothalamic–pituitary function Proc Natl Acad Sci USA 1997 94: 1023–1028.
Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS . Leptin accelerates the onset of puberty in normal female mice J Clin Invest 1997 99: 391–395.
Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA . Leptin is metabolic gate for the onset of puberty in the female rat Endocrinology 1997 138: 855–858.
Plant TM, Durrant AR . Circulating leptin does not appear to provide a signal for triggering the initiation of puberty in the male rhesus monkey (Macaca mulatta) Endocrinology 1997 138: 4505–4508.
Urbanski HF, Pau K-YF . A biphasic developmental pattern of circulating leptin in the male rhesus macaque (Macaca mulatta) Endocrinology 1998 139: 2284–2286.
Mann DR, Akinbami MA, Gould KG, Castracane VD . A longitudinal study of leptin during development in the male rhesus monkey: the effect of body composition and season on circulating leptin levels Biol Reprod 2000 62: 285–291.
Cunningham MJ, Clifton DK, Steiner RA . Leptin's action on the reproductive axis: perspectives and mechanism Biol Reprod 1999 60: 216–222.
Saad MF, Khan A, Sharma A, Michael R, Riad-Gabriel MG, Boyadjian R, Jinagouda SD, Steil GM, Kamdar V . Physiological insulinemia acutely modulates plasma leptin Diabetes 1998 47: 544–549.
Utriainen T, Malmstrom R, Makimattila S, Yki-Jarvinen H . Supraphysiological hyperinsulinemia increases plasma leptin concentrations after 4 h in normal subjects Diabetes 1996 45: 1364–1366.
Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J . Transient increase in obese gene expression after food intake or insulin administration Nature 1995 377: 527–529.
Koopmans SJ, Frolich M, Gribnau EH, Westendrop RG, DeFronzo RA . Effect of hyperinsulinemia on plasma leptin concentrations and food intake in rats Am J Physiol 1998 274: E998–E1001.
Bradley RL, Cheatham B . Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes Diabetes 1999 48: 272–278.
Leonhardt W, Horn R, Brabant G, Breidert M, Temelkova-Kurktschiev TH, Fucker K, Hanefeld M . Relationship of free and specifically bound leptin to insulin secretion in patients with impared glucose tolerance (IGT) Exp Clin Endocrinol Diabet 1999 107: 46–52.
Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry RR, Mudalair SR, Olefsky J, Caro JF . Acute and chronic effect of insulin on leptin production in humans—studies in vivo and in vitro Diabetes 1996 45: 699–701.
Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF . Response of leptin to short term and prolonged overfeeding in humans J Clin Endocrinol Metab 1996 81: 4162–4165.
Kolaczynski JW, Considine RV, Ohannesian JP, Marco C, Opentanova I, Nyce MR, Myint M, Caro JF . Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but no ketones themselves Diabetes 1996 45: 1511–1515.
Vidal HD, Auboeuf D, De Vos P, Staels B, Riou JP, Auwerx J, Laville M . The expression of ob gene is not acutely regulated by insulin and fasting in human abdominal subcutaneous adipose tissue J Clin Invest 1996 98: 251–255.
Kulkarni RN, Whang Z-L, Wang R-M, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR . Leptin rapidly suppress insulin release from insulinoma cells, rat and human islets and, in vivo, in mice J Clin Invest 1997 100: 2729–2736.
D'Adamo MA, Buongiorno E, Maroccia F, Leonetti F, Barbetti A, Giaccari D, Zorretta G, Tamburrano G, Sbraccia P . Increased OB gene expression leads to elevated plasma leptin concentrations in patients with chronic primary hyperinsulinemia Diabetes 1998 47: 1625–1629.
Popovic V, Micic D, Danjanovic S, Zoric S, Djurovic M, Obradovic S, Petakov M, Dieguez, Casanueva FF . Serum leptin and insulin concentrations in patients with insulinoma before and after surgery Eur J Endocrinol 1998 138: 86–88.
Havel PJ, Uriu-Hare JY, Liu T, Stanhope KL, Stern JS, Keen CL, Ahren B . Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats; reveral by insulin Am J Physiol 1998 43: R1482–R1498.
Kieffer T, Habener JF . The adipoinsular axis: effects of leptin on pancreatic b-cells Am J Physiol 2000 278: E1–E14.
Pagano C, Englaro P, Granzotto M, Blum WF, Sagrillo E, Ferretti E, Federspil E, Vettor R . Insulin induces rapid changes of plasma leptin in lean but not in genetically obese (fa/fa) rats Int J Obes Relat Metab Disord 1997 21: 614–618.
Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M . Plasma leptin and insulin relationships in obese and nonobese humans Diabetes 1996 45: 695–698.
Fehmann H-C, Peiser C, Bode H-P, Stamm M, Staats P, Hedetoft C, Lang RE, Goke B . Leptin: a potent inhibitor of insulin secretion Peptides 1997 18: 1267–1273.
Poitout V, Rauault C, Guerre-Millo M, Reach G . Does leptin regulate insulin secretion? Diabetes Metab 1998 24: 313–318.
Russell CD, Petersen RN, Rao SP, Ricci MR, Prasad A, Zhang Y, Brolin RE, Fried SK . Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone Am J Physiol 1998 275: E507–E515.
Poitout V, Rauault C, Guerre-Millo M, Briaud I, Reach G . Inhibition of insulin secretion by leptin in normal rodent islets of Langerhans Endocrinology 1998 139: 822–826.
Emilsson V, Liu Y, Cawthorne MA, Morton NM, Devenport M . Expression and functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion Diabetes 1997 46: 313–316.
Kieffer T, Scott-Heller R, Leech CA, Holz G, Habener JF . Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic b-cells Diabetes 1997 46: 1087–1093.
Seufert J, Kieffer TJ, Leech CA, Holz GG, Moritz W, Ricordi C, Habener JF . Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus J Clin Endocrinol Metab 1999 84: 670–676.
Ceddia RB, William WNJ, Carpinelli AR, Curi R . Modulation of insulin secretion by leptin Gen Pharmac 1999 32: 2323–2327.
Tanizawa Y . Direct stimulation of basal insulin secretion by physiological concentrations of leptin in pancreatic b cells Endocrinology 1997 138: 4513–4516.
Leclercq-Meyer V, Malaisse WJ . Failure of human and mouse leptin to affect insulin, glucagon and somatostatin secretion by the perfused rat pancreas at physiological glucose concentrations Mol Cell Endocrinol 1998 141: 111–118.
Leclercq-Meyer V, Considine RV, Sener A, Malaisse WJ . Does leptin receptor play a functional role in the endocrine pancreas? Biochem Biophys Res Commun 1996 239: 794–798.
Chen N-G, Swick AG, Romsos DR . Leptin constrains acetylcholine induced insulin secretion from pancreatic islets of ob/ob mice J Clin Invest 1997 100: 1174–1179.
Cawthorne MA, Morton NM, Pallet AL, Liu YL, Emilsson V . Peripheral metabolic actions of leptin Proc Nutr Soc 1998 57: 449–453.
Walder K, Filipps A, Clark S, Zimmet P, Collier GR . Leptin inhibits insulin binding in isolated rat adipocytes J Endocrinol 1997 155: R5–R7.
Muller G, Ertl J, Gerl M, Preibisch G . Leptin impairs metabolic actions of insulin on isolated rat adipocytes J Biol Chem 1997 272: 10585–10593.
Zierath JR, Frevert EU, Ryder JW, Berggren P-O, Kahn BB . Evidence against a direct effect of leptin on glucose transport in skeletal muscle and adipocytes Diabetes 1998 47: 1–4.
Ranganathan S, Ciaraldi TP, Henry RR, Mudaliar S, Kern PA . Lack of effect of leptin on glucose transport, lipoprotein lipase, and insulin action in adipose and muscle cells Endocrinology 1998 139: 2509–2513.
Clement K, Lahlou N, Rutz J . Association of poorly controlled diabetes with low serum leptin in morbid obesity Int J Obes Relat Metab Disord 1997 21: 556–561.
Mantzoros CS, Liolias AD, Tritos NA, Kaklamani VG, Doulgerakis DE, Griveas I, Moses A, Flier JS . Circulating insulin concentrations, smoking, and alcohol intake are important independent predictor of leptin in young healthy men Obes Res 1998 6: 179–186.
Segal K, Land M, Klein S . Relationship between insulin sensitivity and plasma leptin concentration inlean and obese men Diabetes 1996 45: 987–991.
Dunaif A . Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis Endocr Rev 1997 18: 774–800.
Blache D, Tellam RL, Chagas LM, Blackberry MA, Vercoe PE, Martin GB . Level of nutrition affects leptin concentrations in plasma and cerebrospinal fluid in sheep J Endocrinol 2000 165: 625–637.
Mizuno T, Bergen H, Kleopoulos S, Bauman WA, Mobbs CV . Effects of nutritional status and ageing on leptin gene expression in mice: importance of glucose Horm Metab Res 1996 28: 679–684.
Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, Stern JS, Havel PJ . Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes Endocrinology 1998 139: 551–558.
Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ . Acute stimulation of glucose metabolism in mice by leptin treatment Nature 1997 389: 374–377.
Muoio DM, Dohm GL, Tapscott EB, Coleman RA . Leptin opposes insulin's effects on fatty acid partitioning in muscle isolated from obese ob/ob mice Am J Physiol 1999 276: E913–E921.
Cohen B, Novick D, Rubinstein M . Modulation of insulin activities by leptin Science 1996 274: 1185–1188.
Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB . Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase Nature 2002 415: 339–343.
Muoio DM, Dohm GL, Fiedorek FT, Tapscott EB, Coleman RA . Leptin directly alters lipid partitioning in skeletal muscle Diabetes 1997 46: 1360–1363.
Burcelini RSK, Li J, Tannenbaum GS, Charron MJ, Friedman JM . Acute intravenous leptin infusion increases glucose turnover but not skeletal muscle glucose uprtake in ob/ob mice Diabetes 1999 48: 1264–1269.
Solini A, Bonora E, Bonadonna R, Castellino P, DeFronzo RA . Protein metabolism in human obesity: relationship with glucose and lipid metabolism and with visceral adipose tissue J Clin Endocrinol Metab 1997 82: 2552–2558.
Ceddia RB, William WNJ, Curi R . Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle; evidence for an effect of leptin on glucose uptake and decarboxylation Int J Obes Relat Metab Disord 1999 23: 75–82.
Liu Y-L, Emilsson V, Cawthorne MA . Leptin inhibits glycogen synthesis in the isolated soleus muscle of obese (ob/ob) mice FEBS Lett 1997 411: 351–355.
Berti L, Kellerer M, Capp E, Haring HU . Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes; evidence for a PI3-kinase mediated effect Diabetologia 1997 40: 606–609.
Berti L, Gammeltoft S . Leptin stimulates glucose uptake in C2C12 muscle cells by activation of ERK2 Mol Cell Endocrinol 1999 157: 121–130.
Nowak K, Mackowiak P, Nogowski L, Szkudelski T, Malendowicz . Acute action on insulin blood level and liver insulin receptor in the rat Life Sci 1998 63: 1347–1352.
Zhao AZ, Shinohara MM, Huang D, Schimizu M, Eldar-Finkelman H, Krebs EG, Beavo JA, Bornfeldt KE . Leptin induces insulin-like signaling that antagonises cAMP elevation by glucagon in hepatocytes J Biol Chem 2000 275: 11348–11354.
Ahren B, Havel PJ . Leptin inhibits insulin secretion induced by cellular cAMP in pancreatic B cell line (INS-1 cells) Am J Physiol 1999 277: R959–R966.
Considine RV, Cooksey RC, Williams LB, Fawcett RL, Zhang P, Ambrosius WT, Whitfield RM, Jones R, Inman M, Huse J, McClain DA . Hexosamines regulate leptin production in human subcutaneous adipocytes J Clin Endocrinol Metab 2000 85: 3551–3556.
McClain DA . Hexosamines as mediators of nutrient sensing and regulation in diabetes J Diabetes Complications 2002 16: 72–80.
Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L . Role of the glucosamine pathway in fat-induced insulin resistance J Clin Invest 1997 99: 2173–2182.
Virkamaki A, Daniels MC, Hamalainen S, Utriainen T, McClain D, Yki-Jarvinen H . Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance in multiple insulin sensitive tissues Endocrinology 1997 138: 2501–2507.
Zhang P, Klenk ES, Lazzaro MA, Williams LB, Considine RV . Hexosamines regulate leptin production in 3T3-L1 adipocytes through transcriptional mechanisms Endocrinology 2002 143: 99–106.
Ciaraldi TP, Carter L, Nikoulina S, Mudaliar S, McClain DA, Henry RR . Glucosamine regulation of glucose metabolism in cultured human skeletal muscle cells: divergent effects on glucose transport/phosphorylation and glycogen synthase in non-diabetic and type 2 diabetic subjects Endocrinology 1999 140: 3971–3980.
McClain DA, Alexander T, Cooksey RC, Considine RV . Hexosamines stimulate leptin production in transgenic mice Endocrinology 2000 141: 1999–2002.
Rossetti L . Perspective: Hexosamines and nutrient sensing Endocrinology 2000 141: 1922–1925.
Luiken JJFP, Dyck DJ, Han X-X, Tandon NN, Arumugam Y, Glatz JFC, Bonen A . Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane Am J Physiol Endocrinol Metab 2002 282: E491–495.
Berk PD, Zhou SL, Bradbury M, Stump D, Kiang CL, Isola LM . Regulated membrane transport of free fatty acids in adipocytes: role in obesity and non-insulin dependent diabetes mellitus Trans Am Clin Climatol Assoc 1996 108: 26–40 [discussion 41–43]
Zhou SL, Stump D, Sorrentino D, Potter BJ, Berk PD . Adipocyte differentiation of 3T3-L1 cells involves augmented expression of a 43-kDa plasma membrane fatty acid-binding protein J Biol Chem 1992 267: 14456–14461.
Randle PJ, Garland PB, Hales CN, Newsholme EA . The glucose-fatty acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus Lancet 1963 1: 785–789.
Ceddia RB, William WN Jr, Curi R . The response of skeletal muscle to leptin Front Biosci 2001 6: D90–D97.
Bryson JM, Phuyal JL, Swan V, Caterson ID . Leptin has acute effects on glucose and lipid metabolism in both lean and gold thioglucose-obese mice Am J Physiol Endocrinol Metab 1999 277: E417–E422.
Slieker LJ, Sloop KW, Surface PL, Kriauciunas A, LaQuier F, Manetta J, Blue-Valleskey J, Stephens TW . Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP J Biol Chem 1996 271: 5301–5304.
Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, Rascher W, Teller W, Tornqvist H, Hauner H . Insulin and cortisol promote leptin production in cultured human fat cells Diabetes 1996 45: 1435–1438.
Tempel DL, Leibowitz SF . Adrenal steroid receptors: interactions with brain neuropeptide systems in relation to nutritient intake and metabolism J Neuroendocrinol 1994 6: 479–501.
Tataranni P, Larsen DE, Snitker S, Young JB, Flatt JP, Ravussin E . Effects of glucocorticoids on energy metabolism and food intake in humans Am J Physiol 1996 271: E317–E325.
Dagogo-Jack S, Selke G, Melson AK, Newcomer JW . Robust leptin secretory responses to dectamethasone in obese subjects J Clin Endocrinol Metab 1997 82: 3230–3233.
Miell JP, Englaro P, Blum WF . Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects Horm Metab Res 1996 28: 704–707.
Solano JM, Jacobson L . Glucocorticoids reverse leptin effects on food intake and body fat in mice without increasing NPY mRNA Am J Physiol 1999 E708–E716.
Kiess W, Englaro P, Hanitsch S, Rascher W, Attanasio A, Blum WF . High leptin concentrations in serum of very obese children are further stimulated by dexamethasone Horm Metab Res 1996 28: 708–710.
De Vos P, Saladin R, Auwerx J, Staels B . Induction of ob gene expression by corticosteroids is accompanied by body weight loss and reduced food intake J Biol Chem 1995 270: 15958–15961.
De Vos P, Lefebvre AM, Shrivo I, Fruchart JC, Auwerx J . Glucocorticoids induce the expression of the leptin gene through a non-classical mechanism of transcriptional activation Eur J Biochem 1998 253: 619–626.
Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte DJ, Woods SC, Seeley RJ, Weigle DS . Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice Diabetes 1996 45: 531–535.
Considine RV . Weight regulation, leptin growth hormone Horm Res 1997 48: 116–121.
Nyomba BLG, Johnson M, Berard L, Murphy LJ . Relationship between serum leptin and the insulin-like growth factor-I system in humans Metab Clin Exp 1999 48: 840–844.
Carro E, Senaris R, Considine RV, Casanueva FF, Dieguez C . Regulation of in vivo growth hormone secretion by leptin Endocrinology 1997 138: 2203–2206.
Carro E, Senaris RM, Seoane LM, Frohman LA, Arimura A, Casanueva FF, Dieguez C . Role of growth hormone (GH)-releasing hormone and somatostatin on leptin-induced GH secretion Neuroendocrinology 1999 69: 3–10.
Tannenbaum GS, Gurd W, Laponte M . Leptin is a potent stimulator of spontaneous pulsatile growth hormone (GH) secretion and the GH response to GH-releasing hormone Endocrinology 1998 139: 3871–3875.
Dyer CJ, Simmons JM, Matteri L, Keisler DH . Leptin receptor mRNA is expressed in ewe anterior pituitary and adipose tissue and is differentially expressed in hypothalamic regions of well-fed and feed-restricted ewes Domest Anim Endocrinol 1997 14: 119–128.
Roh SH, Clarke IJ, Xu RW, Goding JW, Loneragan K, Chen C . The in vitro effect of leptin on basal and growth hormone-releasing secretion from the ovine pituitary gland Neuroendocrinology 1998 68: 361–364.
Williams LM, Adam CL, Mercer JG, Moar KM, Slater D, Hunter L, Findlay PA, Hoggard N . Leptin receptor and neuropeptide Y gene expression in the sheep brain J Neuroendocrinol 1999 11: 165–169.
Barb CR, Yan X, Azain MJ, Kraeling RR, Rampacek GB, Ramsay TG . Recombinant porcine leptin reduces feed intake and stimulates growth hormone secretion in swine Domest Anim Endocrinol 1998 15: 77–86.
Houseknecht KL, Portocarrero CP, Lamenager R, Spurlock ME . Growth hormone regulates leptin gene expresion in bovine adipose tissue: correlation with adipose IGF-1 expression J Endocrinol 2000 164: 51–57.
Rauch F, Westermann F, Englaro P, Blum WF, Schonau E . Serum leptin is suppressed by growth hormone therapy in growth-hormone-deficient children Horm Res 1998 50: 18–21.
Isozaki O, Tsushima T, Miyakawa M, Nozoe Y, Demura H, Seki H . Growth hormone directly inhibits leptin gene expression in viscelar fat tissue in fatty Zucker rats J Endocrinol 1999 161: 511–516.
Chen XL, Hausman DB, Dean RG, Hausman HJ . Hormonal regulation of leptin mRNA expression and preadipcyte recruitment and differentiation in porcine primary cultures and S-V cells Obes Res 1998 6: 164–172.
Wabitsch M, Blum WF, Muche R, Braun M, Hube F, Rascher W, Heinze E, Teller W, Hauner H . Contribution of androgens to the gender difference in leptin production in obese children and adolescents J Clin Invest 1997 100: 808–813.
Boni-Schnetzler M, Gosteli-Peter MA, Moritz W, Froesch ER, Zapf J . Reduced ob mRNA in hypophysectomised rats is not restored by growth hormone (GH), but further suppressed by exogenously administered insulin-like growth factor (IGF) 1 Biochem Biophys Res Commun 1996 225: 296–301.
Boni-Schnetzler M, Hauri C, Zapf J . Leptin is suppressed during infusion of recombinant human insulin-like growth factor I (rhIGFI) in normal rats Diabetologia 1999 42: 160–166.
Janik JE, Cutri BD, Considine RV, Rager HC, Powers GC, Alvord WG, Smith JW II, Gause BL, Kopp WC . Interleukin 1a increases serum leptin concentrations in humans J Clin Endocrinol Metab 1997 82: 3084–3086.
Zumbach MS, Boehme MWJ, Wahl P, Stremmel W, Ziegler R, Nawroth PP . Tumor necrosis factor increases serum leptin levels in humans J Clin Endocrinol Metab 1997 82: 4080–4082.
Matarese G . Leptin and the immune system: how nutritional status influences the immune response Eur Cytokine Netw 2000 11: 7–13.
Mantzoros CS, Moschos S, Avramopoulos L, Kaklamani V, Liolios A, Doulgerakis DE, Griveas I, Katsilambros N, Flier JS . Leptin concentrations in relation to body mass index and the tumour necrosis factor—a system in humans J Clin Endocrinol Metab 1997 82: 3408–3413.
Grunfeld C, Zhao C, Fuller J, Pollock A, Moser A, Friedman J, Feingold KR . Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamster J Clin Invest 1996 97: 2152–2157.
Langhans W, Hrupka B . Interleukins and tumor necrosis factor as inhibitors of food intake Neuropeptides 1999 33: 415–424.
Mantzoros CS, Moschos SJ . Leptin: in search of role(s) in human physiology and pathophysiology Clin Endocrinol 1998 49: 551–567.
Shintani M, Nishimura H, Yonemitsu S, Masuzaki H, Ogawa Y, Hosoda K, Inoue G, Yoshimasa Y, Nakao K . Downregulation of leptin by free fatty acids in rat adipocytes: effects of triascin C, palmitate, and 2-bromopalmitate Metabolism 2000 49: 326–330.
Fain JN, Coronel EC, Beauchamp MJBS . Expression of leptin and β2-adrenergic receptors in rat adipose tissue in altered thyroid states Biochem J 1997 322: 145–150.
Yoshida T, Monkawa T, Hayashi M, Saruta T . Regulation of expression of leptin mRNA and secretion of leptin by thyroid hormone in 3T3-L1 adipocyte Biochem Biophys Res Commun 1997 232: 822–826.
Mantzoros CS, Rosen HN, Greenspan SL, Flier JS, Moses AC . Short-term hyperthyroidism has no effect on leptin levels in man J Clin Endocrinol Metab 1997 82: 497–499.
Sesmilo G, Casamitjana R, Halperin I, Gomis R, Vilardell E . Role of thyroid hormones on serum leptin levels Eur J Endocrinol 1998 139: 428–430.
Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS . Leptin levels reflect body lipid content in mice; evidence for diet-induced resistance to leptin action Nat Med 1995 1: 1311–1314.
Iida M, Murakami T, Yamada M, Sei M, Kuwajima M, Mizuno A, Noma Y, Aono T, Shima K . Hyperleptinaemia in chronic renal failure Horm Metab Res 1996 28: 724–727.
Merabet E, Dagogo-Jack S, Coyne DW, Klein S, Santiago JV, Hmiel SP, Landt M . Increased plasma leptin concentration in end-stage renal disease J Clin Endocrinol Metab 1997 82: 847–850.
Leroy P, Dessolin S, Villageois P, Moon BM, Friedman JM, Ailhaud G, Dani C . Expression of ob gene in adipose cells: regulation by insulin J Biol Chem 1996 271: 2365–2368.
Kolaczynski JW, Golstein BJ, Considine RV . Dexamethasone, ob gene and leptin in humans: effect of exogenous hyperleptinaemia J Clin Endocrinol Metab 1997 82: 3895–3897.
Papaspyrou-Rao S, Schneider SH, Petersen RN, Fried SK . Dexamethosone increases leptin expression in humans in vivo J Clin Endocrinol Metab 1997 82: 1635–1637.
Zhang HH, Kumara S, Barnett AH, Eggo MC . Tumor necrosis factor-α exerts dual effect on human adipose leptin synthesis and release Mol Cell Endocrinol 2000 159: 79–88.
Qian H, Barb CR, Compton MM, Hausman GJ, Azain MJ, Kraeling RR, Bailie CA . Leptin mRNA expression and serum leptin concentrations as influenced by age, weight and estradiol in pigs Domest Anim Endocrinol 1999 16: 135–143.
Ghorbani M, Himms-Hagen J . Treatment with CL 316,242 a β3-adrenoceptor agonist, reduces serum leptin in rats with aging-associated obestiy but not in Zucker rats with genetic (fa.fa) obesity Int J Obes Relat Metab Disord 1998 22: 63–65.
Gettys TW, Harkness PJ, Watson PM . The β3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes Endocrinology 1996 137: 4054–4057.
Hodge AM, Westerman RA, de Courten MP, Collier GR, Zimmet PZ . Is leptin sensitivity the link between smoking cessation and weight gain Int J Obes Relat Metab Disord 1997 21: 50–53.
Wei M, Stern MP, Haffner SM . Serum leptin levels in Mexican Americans and non-Hispanic whites: association with body mass index and cigarette smoking Ann Epidemiol 1997 7: 81–86.
Miyata G, Meguid MM . Is leptin involved in the acute anorectic effect of nicotine? Nutrition 2000 16: 141–142.
Pallett AL, Morton NM, Cawthorne MA, Emilsson V . Leptin inhibits insulin secretion and reduces insulin mRNA levels in rat isolated pancreatic cells Biochem Biophys Res Commun 1997 238: 267–270.
Ishida K, Murakami T, Yamada M, Sei M, Kuwajima M, Shima K . Leptin suppresses basal insulin secretion from rat pancreatic islets Regul Peptides 1997 70: 179–182.
Roduit R, Thorens B . Inhibition of glucose-induced insulin secretion by long-term preexposure of pancreatic islets to leptin FEBS Lett 1997 425: 179–182.
Glick ME . Fat, bodyweight regulated by newly discovered hormone 1996 URL http://www.rockefeller.edu/pubinfo/ob.rel.nr.html Date accessed March 26, 1998
Acknowledgements
This work was supported in part by the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Margetic, S., Gazzola, C., Pegg, G. et al. Leptin: a review of its peripheral actions and interactions. Int J Obes 26, 1407–1433 (2002). https://doi.org/10.1038/sj.ijo.0802142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802142
Keywords
This article is cited by
-
Leptin and leptin receptor expression as biomarkers for breast cancer: a retrospective study
BMC Cancer (2023)
-
Global analysis of the association between pig muscle fatty acid composition and gene expression using RNA-Seq
Scientific Reports (2023)
-
Association between serum interleukin-6, leptin and insulin in gestational diabetes mellitus – a cross- sectional study
Journal of Diabetes & Metabolic Disorders (2023)
-
Immunohistochemical identification and assessment of the location of leptin, visfatin and chemerin in the liver of men with different body mass index
BMC Gastroenterology (2022)
-
Leptins: association and clinical correlation in pre-diabetics
International Journal of Diabetes in Developing Countries (2022)