Abstract
BACKGROUND: Four animal models of virus-induced obesity including adiposity induced by an avian adenovirus have been described previously. This is the first report of adiposity induced in animals by a human virus.
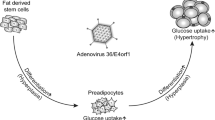
OBJECTIVE: We investigated the adiposity promoting effect of a human adenovirus (Ad-36) in two different animal models.
DESIGN: Due to the novel nature of the findings we replicated the experiments using a chicken model three times and a mammal model once. In four separate experiments, chickens and mice were inoculated with human adenovirus Ad-36. Weight matched groups inoculated with tissue culture media were used as non-infected controls in each experiment. Ad-36 inoculated and uninfected control groups were housed in separate rooms under biosafety level 2 or better containment. The first experiment included an additional weight matched group of chickens that was inoculated with CELO (chick embryo lethal orphan virus), an avian adenovirus. Food intakes and body weights were measured weekly. At the time of sacrifice blood was drawn and visceral fat was carefully separated and weighed. Total body fat was determined by chemical extraction of carcass fat.
RESULTS: Animals inoculated with Ad-36 developed a syndrome of increased adipose tissue and paradoxically low levels of serum cholesterol and triglycerides. This syndrome was not seen in chickens inoculated with CELO virus. Sections of the brain and hypothalamus of Ad-36 inoculated animals did not show any overt histopathological changes. Ad-36 DNA could be detected in adipose tissue, but not skeletal muscles of randomly selected animals for as long as 16 weeks after Ad-36 inoculation.
CONCLUSIONS: Data from these animal models suggest that the role of viral disease in the etiology of human obesity must be considered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL . Increasing prevalence of overweight among US adults JAMA 1994 272: 205–211.
Pi-Sunyer FX . Medical hazards of obesity Ann Intern Med 1993 119: 655–660.
Bray GA . Syndromes of hypothalamic obesity in man Ped Ann 1984 13: 525–536.
Lyons MJ, Faust IM, Hemmes RB, Buskirk DR, Hirsch J, Zabriskie JB . A virally induced obesity syndrome in mice Science 1982 216: 82–85.
Bernard A, Zwingelstein G, Meister RR, Fabian-Wild T . Hyperinsulinemia induced by canine distemper virus infection of mice and its correlation with the appearance of obesity Comp Biochem Physiol 1988 91B: 691–696.
Bernard A, Fevre-Montange M, Giraudon P, Hardin H, Fabian-Wild T, Confavreux C, Belin MF . Brain structures selectively targeted by canine distemper virus in a mouse model infection J Neuropath Exp Neurol 1993 52: 471–480.
Nagashima K, Zabriskie JB, Lyons MJ . Virus induced obesity in mice. Association with a hypothalamic lesion J Neuropathol Exp Neurol 1992 51: 101–109.
Carter JK, Ow CL, Smith RE . Rous-associated virus type 7 induces a syndrome in chickens characterized by stunting and obesity Infect Immunol 1983 39: 410–422.
Carter JK, Garlich JD, Donaldson WT, Smith RE . Influence of diet on a retrovirus induced obesity and stunting syndrome Avian Dis 1983 27: 317–322.
Gosztonyi G, Ludwig H . Borna disease: neuropathology and pathogenesis Curr Top Microbiol Immunol 1995 190: 39–73.
Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA . Effect of adenovirus infection on adiposity in chickens Vet Microbiol 1992 31: 101–107.
Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL . Association of adenovirus infection with human obesity Obes Res 1997 5: 464–469.
Wigand R, Gelderblom H, Wadell G . New human adenovirus (candidate adenovirus 36), a novel member of subgroup D Arch Virol 1980 64: 225–233.
De Jong JC, Bijlsma K, Wermenbol AG, Verweij-Uijterwaal MW, Van Der Avoort HGAM, Wood DJ, Bailey AS, Osterhaus ADME . Detection, typing and subtyping of enteric adenoviruses 40 and 41 from fecal samples and observation of changing incidences of infections with types and subtypes J Clin Microbiol 1993 31: 1562–1569.
De Jong JC, Wigand R, Wadell G, Keller D, Muzeric CJ, Wermenbc AG, Schaap GJP . Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D J Med Virol 1981 7: 105–118.
De Jong JC, Wigand R, Adrian Th, Hierholzer JC, Kapsenberg JG, Muzerie CJ, Wermenbol AG . Adenovirus 38: a new human adenovirus species of subgenus D Intervirology 1984 22: 164–169.
Wigand R, Adrian T, Bricout F . A new human adenovirus of subgenus D: candidate adenovirus type 42 Arch Virol 1987 94: 283–286.
Hirholzer JC, Wigand R, Anderson LJ, Adrian T, Gold JWM . Adenoviruses from patients with AIDS: a plethora of serotypes and a description of five new serotypes of subgenus D (Types 43–47) J Infect Dis 1988 158: 804–813.
Kolesar J, Allen P, Doran CM . Direct quantification of HIV-1 RNA by capillary electrophoresis with laser-induced fluorescence J Chromatogr 1997 B697: 189–194.
Yates VJ, Rhee YO, Fry DE, El Mishad AM, McCormick KJ . The presence of avian adenoviruses and adeno-associated viruses in healthy chickens Avian Dis 1975 20: 146–152.
Dixon RM, Borden EC, Keim NL, Anderson S, Spennetta TL, Torrney DC, Shrago E . Decreases in serum high density lipoprotein cholesterol and total cholesterol resulting from naturally produced and recombinant DNA derived leukocyte interferon Metabolism 1984 33: 400–404.
Dhurandhar NV, Sheele J, Israel BA, Atkinson RL . Adenovirus that induces adiposity in animals also influences in-vitro differentiation of preadipocytes Obes Res 1999 7: 39S.
Atkinson RL, Dhurandhar NV, Allison DB, Bowen R, Israel BA . Evidence for an association of an obesity virus with human obesity at three sites in the United States Int J Obes Relat Metab Disord 1998 22: S57.
Acknowledgements
We gratefully acknowledge Drs Geoffrey Letchworth and Lisa Krugner-Higby for advice on virological aspects and Sharon Gathright, Dr Tami Wolden-Hanson and Kathleen Taylor for laboratory assistance. Supported by funds from the Wisconsin Alumni Research Foundation's Beers–Murphy Clinical Nutrition Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhurandhar, N., Israel, B., Kolesar, J. et al. Increased adiposity in animals due to a human virus. Int J Obes 24, 989–996 (2000). https://doi.org/10.1038/sj.ijo.0801319
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801319
Keywords
This article is cited by
-
Inflammatory cytokines and chemokines in obese adolescents with antibody against to adenovirus 36
Scientific Reports (2023)
-
Adenovirus 36 prevalence and association with human obesity: a systematic review
International Journal of Obesity (2021)
-
Obesity and Diabetes in an Arab population: Role of Adenovirus 36 Infection
Scientific Reports (2020)
-
Adenovirus 36 seropositivity is related to obesity risk, glycemic control, and leptin levels in Chilean subjects
International Journal of Obesity (2020)
-
A personal look at the past and future of obesity science
European Journal of Clinical Nutrition (2020)