Abstract
OBJECTIVE: We examined the relationship of adiposity to pituitary–adrenal responses to corticotrophin-releasing hormone (CRH) in men and postmenopausal women, controlling for the influence of depression.
DESIGN: Studies of CRH responses, cortisol metabolite levels and depression scores in relation to adiposity in men and postmenopausal women.
SUBJECTS: Thirteen men: age (median, interquartile range) 62 y (52–63), body mass index (BMI) 29.0 kg/m2 (26.3–33.1), waist circumference (waist) 105 cm (97–111), waist:hip ratio (WHR) 1.03 (0.98–1.07), subscapular to triceps skinfold thickness ratio (STR) 2.0 (1.2–2.4), total body fat (TBF) 25.4 kg (19.8–28.8); and eight women: age 54 y (53–62), BMI 30 kg/m2 (23–41), waist 86 cm (79–117), WHR 0.94 (0.87–1.10), STR 1.0 (0.85–1.07), TBF 35.0 kg (18.7–48.8).
MEASUREMENTS: A standard CRH test was conducted with additional basal samples taken for leptin and interleukin 6 (IL-6). Total urine cortisol metabolites (TCM) and the ratio of urinary cortisol:cortisone (Fm/Em) metabolites were measured. Depression scores were measured by the General Health Questionnaire (GHQ-30) and Hospital Anxiety and Depression Scale (HAD) questionnaire. All subjects completed an overnight dexamethasone suppression test.
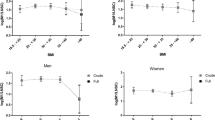
RESULTS: The basal to peak percentage increments (%inc.) in adrenocorticotrophic hormone (ACTH) and cortisol in men correlated directly with STR (ACTH %inc. r=0.70, P<0.01; cortisol %inc. r=0.55, P=0.05); this relationship was independent of depression scores. In women, the ACTH area under incremental curve (AUIC) correlated negatively with STR (r=−0.81, P<0.05). In men, but not in women, there was a significant correlation between GHQ-30 score and ACTH AUIC (r=0.62, P<0.05) and cortisol AUIC (r=0.72, P<0.01). Depression scores were consistently and directly related to indices of obesity and central obesity. There were no significant relationships in either sex between urinary TCM or Fm/Em ratio and BMI, waist, WHR, TBF, STR or CRH responses. The urinary Fm/Em ratio was higher in men than in women (median 0.74 vs 0.66, P<0.05). In men, but not in women, GHQ-30 scores correlated positively with urinary TCM (r=0.57, P=0.05) and HAD-depression scores were inversely related to the urine Fm/Em ratio (r=−0.65, P<0.05). All subjects suppressed normally with dexamethasone.
CONCLUSIONS: Cortisol metabolite levels were increased in depression in men, but were not related to adiposity in either sex. We demonstrate that central obesity in men, but not postmenopausal women, is associated with an enhanced pituitary–adrenal response to CRH and that this relationship is independent of depression score.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kopelman PG, Grossman A, Lavender P, Besser GM, Rees LH, Coy D . The cortisol response to corticotrophin-releasing factor is blunted in obesity Clin Endocrinol Oxf 1988 28 (1): 15–18.
Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi L, Flamia R, Labate AM, Barbara L . The hypothalamic–pituitary–adrenal axis in obese women with different patterns of body fat distribution J Clin Endocrinol Metab 1993 77 (2): 341–346.
Boushaki FZ, Rasio E, Serri O . Hypothalamic–pituitary–adrenal axis in abdominal obesity: effects of dexfenfluramine Clin Endocrinol Oxf 1997 46 (4): 461–466.
Weaver JU, Kopelman PG, McLoughlin L, Forsling ML, Grossman A . Hyperactivity of the hypothalamo–pituitary–adrenal axis in obesity: a study of ACTH, AVP, beta-lipotrophin and cortisol responses to insulin-induced hypoglycaemia Clin Endocrinol Oxf 1993 39 (3): 345–350.
Weyer C, Heise T, Gudat U, Kimmerle R, Santen R, Schlag-hecke R, Spraul M, Berger M . Integrity of the hypothalamo–pituitary–adrenal axis in morbid human obesity Am J Physiol 1997 4: 397–401.
Bjorntorp P . The regulation of adipose tissue distribution in humans Int J Obes Relat Metab Disord 1996 20 (4): 291–302.
Costa A, Poma A, Martignoni E, Nappi G, Ur E, Grossman A . Stimulation of corticotrophin-releasing hormone release by the obese (ob) gene product, leptin, from hypothalamic explants Neuroreport 1997 8 (5): 1131–1134.
Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS . Leptin inhibition of the hypothalamic–pituitary–adrenal axis in response to stress Endocrinology 1997 138 (9): 3859–3863.
Bornstein SR, Uhlmann K, Haidan A, Ehrhart Bornstein M, Scherbaum WA . Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly Diabetes 1997 46 (7): 1235–1238.
Tsigos C, Papanicolaou DA, Defensor R, Mitsiadis CS, Kyrou I, Chrousos GP . Dose effects of recombinant human interleukin-6 on pituitary hormone secretion and energy expenditure Neuroendocrinology 1997 66 (1): 54–62.
van der Meer MJ, Hermus AR, Pesman GJ, Sweep CG . Effects of cytokines on pituitary beta-endorphin and adrenal corticosterone release in vitro Cytokine 1996 8 (3): 238–247.
van der Meer MJ, Sweep CG, Rijnkels CE, Pesman GJ, Tilders FJ, Kloppenborg PW, Hermus AR . Acute stimulation of the hypothalamic–pituitary–adrenal axis by IL-1 beta, TNF alpha and IL-6: a dose response study J Endocrinol Invest 1996 19 (3): 175–182.
Tarnopolsky A, Hand DJ, McLean EK, Roberts H, Wiggins RD . Validity and uses of a screening questionnaire (GHQ) in the community Br J Psychiatry 1979 134: 508–515.
Raven PW, Taylor NF . Sex differences in the human metabolism of cortisol Endocr Res 1996 22 (4): 751–755.
Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P . Cortisol secretion in relation to body fat distribution in obese premenopausal women Metabolism 1992 41 (8): 882–886.
Amsterdam JD, Maislin G, Winokur A, Berwish N, Kling M, Gold P . The CRH stimulation test before and after clinical recovery from depression J Affect Disord 1988 14 (3): 213–222.
Holsboer F, von Bardeleben U, Buller R, Heuser I, Steiger A . Stimulation response to corticotropin-releasing hormone (CRH) in patients with depression, alcoholism and panic disorder Horm Metab Res Suppl 1987 16: 80–88.
Rosmond R, Lapidus L, Marin P, Bjorntorp P . Mental distress, obesity and body fat distribution in middle-aged men Obes Res 1996 4 (3): 245–252.
Solano MP, Kumar M, Fernandez B, Jones L, Goldberg RB . Hypothalamic–pituitary–adrenal axis activity in obese men. Proceedings of American Endocrine Society 80th Annual Meeting: Endo 98. pp. 2–551.
Strain GW, Zumoff B, Strain JJ, Levin J, Fukushima DK . Cortisol production in obesity Metabolism 1980 29 (10): 980–985.
Rosmond R, Dallman MF, Bjorntorp P . Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities [see comments] J Clin Endocrinol Metab 1998 83 (6): 1853–1859.
Kopelman PG, Grossman A (eds) . Clinical Endocrinology. 2nd ed. Oxford: Blackwell Science Ltd, 1998; 68, The Endocrinology of Obesity, pp. 952–956.
Andrew R, Phillips DIW, Walker BR . Obesity and gender influence cortisol secretion and metabolism in man J Clin Endocrinol Metab 1998 83 (5): 1806–1809.
Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH . Cortisol metabolism in human obesity: impaired cortisone→cortisol conversion in subjects with central adiposity J Clin Endocrinol Metab 1999 84 (3): 1022–1027.
Raven PW, Taylor NF . 11Beta-HSD and 17beta-HSD as biological markers of depression: sex differences and correlation with symptom severity Endocr Res 1998 24 (3–4): 659–662.
Weaver JU, Taylor NF, Monson JP, Wood PJ, Kelly WF . Sexual dimorphism in 11-beta hydroxysteroid dehydrogenase activity and its relation to body fat distribution and insulin sensitivity; a study in hypopituitary subjects Clin Endocrinol 1998 49: 13–20.
Bujalska IJ, Kumar S, Stewart PM . Does central obesity reflect “Cushing's disease of the omentum”? Lancet 1997 349 (9060): 1210–1213.
Crofford LJ, Kalogeras KT, Mastorakos G, Magiakou MA, Wells J, Kanik KS, Gold PW, Chrousos GP, Wilder RL . Circadian relationships between interleukin (IL)-6 and hypothalamic–pituitary–adrenal axis hormones: failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis J Clin Endocrinol Metab 1997 82 (4): 1279–1283.
Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G . An assessment of hypothalamo–pituitary–adrenal axis functioning in non-depressed, early abstinent alcoholics Psychoneuroendocrinology 1996 21 (3): 263–275.
Inder WJ, Joyce PR, Ellis MJ, Evans MJ, Livesey JH, Donald RA . The effects of alcoholism on the hypothalamic–pituitary–adrenal axis: interaction with endogenous opioid peptides Clin Endocrinol Oxf 1995 43 (3): 283–290.
Magiakou MA, Mastorakos G, Webster E, Chrousos GP . The hypothalamic–pituitary–adrenal axis and the female reproductive system Ann N Y Acad Sci 1997 816: 42–56.
Vamvakopoulos NC, Chrousos GP . Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction J Clin Invest 1993 92 (4): 1896–1902.
Liu JH, Rasmussen DD, Rivier J, Vale W, Yen SS . Pituitary responses to synthetic corticotropin-releasing hormone: ab-sence of modulatory effects by estrogen and progestin Am J Obstet Gynecol 1987 157 (6): 1387–1391.
Acknowledgements
We would like to achnowledge the financial assistance given by the British Diabetic Association (BDA). This research was funded by a BDA Group Support grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katz, J., Taylor, N., Goodrick, S. et al. Central obesity, depression and the hypothalamo–pituitary–adrenal axis in men and postmenopausal women. Int J Obes 24, 246–251 (2000). https://doi.org/10.1038/sj.ijo.0801122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801122
Keywords
This article is cited by
-
The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D)
Molecular Psychiatry (2013)
-
Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: national health and nutrition examination survey 2005-2006
BMC Psychiatry (2011)
-
Awakening Cortisol Response in Lean, Obese, and Reduced Obese Individuals: Effect of Gender and Fat Distribution*
Obesity (2007)
-
Synergistic effects of depressed mood and obesity on long-term cardiovascular risks in 1510 obese men and women: results from the MONICA–KORA Augsburg Cohort Study 1984–1998
International Journal of Obesity (2006)
-
Interleukin-6 production and deregulation of the hypothalamic-pituitary-adrenal axis in patients with major depressive disorders
Endocrine (2006)