Abstract
Unlike most studies on hybridisation between oilseed rape and Brassica rapa, this study focused on hybridisation with oilseed rape as the maternal parent. This is a key cross because, assuming that plastids are inherited maternally, F1-hybrid production with maternal oilseed rape (B. napus) is the only transgene escape route from transplastomic oilseed rape. We investigated such F1-hybrid production in winter oilseed rape co-cultivated with weedy B. rapa at three plant densities each with two proportions of the different species. The paternity of the progeny produced on oilseed rape was assessed, and several fitness parameters were determined in oilseed rape mother plants in order to correlate hybridisation and plant competition. At higher density, the vegetative fitness per mother plant decreased significantly, but the density only affected the frequency of F1-hybrids significantly (a decrease) in the treatment with equal proportions of each species. As to the proportions, at higher B. napus frequencies, there were fewer F1-hybrids per mother plant and a significant increase in most biomass components. Thus, B. rapa was the stronger competitor in its effect on both the vegetative and reproductive fitness in B. napus, and the hybridisation frequency. In conclusion, the relative frequency of the two species was a more influential parameter than the density. Hybridisation with B. napus as the female will be most likely at current field densities of B. napus and when B. rapa is an abundant weed.
Similar content being viewed by others
Introduction
Farmers should be able to choose freely among crop types and management forms, – be it genetically modified (GM) or conventional crop production. However, cultivation of GM crops should not affect ecosystems or non-GM farmers' economy in unwanted ways. One of the large obstacles to integrating GM crops into the cropping system is the flow of transgene-containing pollen to the surroundings. It would therefore be of great value to remove transgenes from pollen. In most angiosperms, inheritance of plastids are only or mainly maternal (Mogensen, 1996), and, therefore, integrating transgenes in plastid DNA (producing transplastomic lines) is supposed to decrease transgene dispersal. Consequently, adventitious mixture of transgenes in the harvest from non-GM fields, outcrossing to wild relatives and transgene stacking would be reduced. To date, transplastomic lines have been produced in several plant species (Svab et al, 1990; Khan and Maliga, 1999; Sidorov et al, 1999; Ruf et al, 2001; Skarjinskaia et al, 2003), including oilseed rape (Hou et al, 2003).
Plastid DNA has not been found in the generative and sperm cells of oilseed rape (Corriveau and Coleman, 1988), which is in accordance with the finding that chloroplast DNA inheritance in this species is strictly maternal (Erickson and Kemble, 1990). Therefore, transplastomic oilseed rape would be welcomed, as oilseed rape has a large potential for pollen dispersal. Oilseed rape outcrosses and hybridises readily with other oilseed rape individuals in the neighbourhood and with wild relatives as Brassica rapa and Raphanus raphanistrum (Chèvre et al, 2004). Both wind and insects are pollen vectors, which make pollen-flow hard to control. Besides being cultivated, oilseed rape is a common weed in arable fields with inefficient weed control, and it appears as a volunteer in fields, and as a ruderal plant (Tolstrup et al, 2003). Seed dispersal in oilseed rape can also contribute to transgene dispersal. Seeds are spilled mostly before and at harvest; on average, 5–10% of the production, but in some years up to 50%. The seeds contribute to the seed bank for up to 10–12 years (Tolstrup et al, 2003).
Wild B. rapa has a wide distribution, abundant, for example, in parts of Denmark and southern Sweden. It occurs as a weed in oilseed rape fields, along roadsides and in other disturbed habitats (Landbo et al, 1996). B. rapa is one of the parental diploids of the tetraploid oilseed rape. Owing to this relatedness, the two species are crosscompatible, and concerns have been raised that transgenes would introgress from oilseed rape to B. rapa. The first step in this introgression process would be formation of F1-hybrids. Frequencies of hybridisation with B. rapa as seed parent (♀) has been reported by a number of authors (eg Landbo et al, 1996; Pertl et al, 2002; Warwick et al, 2003), rather than with B. napus as the seed parent (Jørgensen and Andersen, 1994). However, since hybridisation with B. napus as the seed parent is the transgene escape route for transplastomic varieties, it is important to estimate the extent of hybridisation in this cross.
We therefore wanted to throw light on the following questions:
-
1)
Is the F1 production influenced by different proportions and densities of B. napus and B. rapa?
-
2)
Do different proportions and densities change the growth conditions and, consequently, the vegetative and reproductive fitness of B. napus?
We estimated the F1-hybrid production on a winter variety of oilseed rape, cocultivated in the field with weedy B. rapa in two different proportions and three different densities. The F1-hybrids were identified by their genetic fingerprint; progeny harvested on B. napus with B. rapa-specific DNA markers were classified as F1-hybrids. To reveal the effect of plant competition on vegetative and reproductive fitness (eg interspecific hybridisation), several fitness parameters were determined.
Materials and methods
Field trial
Transplastomic oilseed rape varieties were not available, thus we chose the conventional oilseed rape variety Capitol (winter type, produced through the doubled-haploid technique by ‘Cargill’, France). In August, it was sown in a separate plot and vernalised over winter. Seeds of weedy B. rapa consisting of the offspring from a controlled intercross between four wild populations from the eastern part of Zealand, Denmark, were germinated according to treatment 3 described by Landbo and Jørgensen (1997): 96 h temperature cycling of seeds on filter paper in 0.2% KNO3, 16 h at 20°C followed by 8 h at 30°C. Germinated seeds were transplanted into soil filled trays and cultivated in the greenhouse until the 3–4 leaves stage. In the middle of May, both species were transplanted to the experimental plots. The flowering time of the two species was synchronised (buds removed on some B. rapa plants), as they have a considerable overlap in flowering time under natural conditions. Plots were established with oilseed rape and B. rapa mixed in a 3:1 (B. napus:B. rapa) proportion corresponding to a realistic scenario in some Danish oilseed rape fields, and in a 1:1 proportion as a worst-case scenario, corresponding to, for example, a mixed weedy population or a ruderal site.
In agricultural fields, the recommended density of oilseed rape is 60–90 plants/m2. Lower densities increase the risk of yield loss, whereas higher densities increase the risk for elongation growth in the autumn. Under good growth conditions, 40 plants/m2 is acceptable (Danish Agricultural Advisory Service, Århus, Denmark). We chose densities of 16 plants/m2 (25 cm between plants), 44.5 plants/m2 (15 cm between plants) and 100 plants/m2 (10 cm between plants). In total, there were six plots in the experiment: three (plant densities) × two (species proportions). Plants were planted equally spaced at the three plant densities and in specific patterns to ensure that the species proportions were achieved. The plots were rectangular and included 150 B. napus and 50 B. rapa plants in 3:1 proportion, and 50 plants of each species at 1:1 proportion. Plots with the same species proportion, but different plant densities, were separated by 1 m, whereas the two proportions were separated by 10 m of winter wheat. As is normal in oilseed rape fields, plants were sprayed at the bud stage against pollen beetles (Meligethes aeneus). Weeds were controlled manually.
After 11 weeks, the seeds on B. napus were mature, and plants inside the two outermost rows surrounding the plots were harvested. The plants were cut just above the ground and left to dry in open paper-bags. For analysis of paternity, 10 B. napus mother plants from each plot were selected at random, and 50 seeds from each mother plant were germinated, giving 3000 progeny. The vegetative dry biomass, the number of pods, the yield (total seed weight) and the seed number were also determined for the 10 mother plants per plot.
Controlled crosses
To compare the marker frequencies of known F1-hybrids with F1-hybrids from the field, marker analyses of 100 F1-hybrids obtained from controlled crosses between B. napus (♀) × B. rapa (♂) were performed. The controlled crosses were made with 10 B. napus and 100 B. rapa plants from the same intercrossed population used in the field experiment. All B. napus flower buds were emasculated 2–3 days before the opening of the flowers; buds not used were removed. Bumblebees carried out the pollination.
DNA extraction, SSR and Inter-SSR analysis
DNA was extracted from leaves by the simple alkaline extraction technique described by Milligan (1998), with the following modifications: 200 μl 0.5 M NaOH were added to a 1–2 cm2 leaf sample in a micro tube and grinded with two steel beads in a mixer mill. In total, 20 μl of extraction-suspension was transferred to 480 μl storage buffer.
The SSR-PCR was carried out using the procedure described by Szewc-McFadden et al (1996) with modifications as follows: 2 μl extracted DNA, 1 U Taq DNA polymerase (Promega) with 1 × Reaction Buffer A (provided with the enzyme) and 2.0 mM MgCl2. PCR was made with the primer pair B.n. 12A (Szewc-McFadden et al, 1996). PCR amplifications were performed on a Techne Touchgene Thermocycler (Buch & Holm A/S) following the programme described by Szewc-McFadden et al (1996).
The Inter-SSR-PCR was carried out according to the procedure described by Charters et al (1996) with modifications as follows: 5 μl extracted DNA, 1 U Taq DNA polymerase with 1 × Reaction Buffer A and 2.0 mM MgCl2. PCR was made with the degenerate primer 888 (BDB-[CA7]) (Charters et al, 1996). PCR amplifications were performed on a Techne Genius Thermocycler (Buch & Holm A/S) and the programme described by Charters et al (1996) was applied.
After the PCR, 8 μl formamide loading buffer (bromphenol blue xylen cyanol dye solution, Sigma-Aldrich) was added to the reaction products. The samples were heated at 96°C for 5 min and then quickly cooled on ice. Then, 1 μl SSR and 4 μl Inter-SSR product were multiplexed in the loading process, and loaded on a 3% polyacrylamide gel. The PCR-products were electrophoresed and visualised by silver staining after the methods described by Johannessen et al (2002). DNA markers specific to B. rapa were identified by screening 100 B. rapa individuals (the B. rapa test sample) from the same population used in the field, and 100 individuals of the oilseed variety Capitol.
Data analysis
We evaluated the following hypotheses: The composition (proportion) and density of plants are environmental factors changing the competitive conditions. Changes in the competitive conditions are reflected in the vegetative fitness, which has an effect on the reproductive fitness. Both vegetative and reproductive fitness affect hybridisation; therefore, these environmental factors have an effect on the frequency of F1-hybrids per mother plant and the number of F1-hybrids per square-meter.
Data were subjected to analysis of variance (ANOVA, software: SAS version 8.2, SAS Institute Inc., Cary, NC, USA). The analyses were carried out with the dependent variables as follows: the frequency of F1-hybrids per mother plant and the number of F1-hybrids per square-meter. The analyses were also carried out for the pod number, the yield, the seed number and the dry biomass as the dependent variables since these biomass components express the vegetative fitness of the oilseed rape mother plants. The analyses involved the proportion and density of plants in the field as factors. With the frequency of F1-hybrids and the number of F1-hybrids per square-meter as dependent variables, the pod number, yield, seed number and dry biomass were included in the analyses as covariates. Pod number, seed number and dry biomass were also included as covariates when the analyses were made with the dependent variable yield.
Results
Markers for identification of F1-hybrids and calculation of frequencies of F1-hybrids
The fingerprinting techniques (Figure 1) produced three dominant markers, ISSR-1, ISSR-2 and SSR-1, that were specific to the B. rapa population used in this experiment. Since none of the developed markers were monomorphic and homozygous in the 100 individuals of the B. rapa test sample, some F1-hybrids would remain undetected. The three markers specific to B. rapa were not different alleles at the same locus, as all kinds of permutations between the three markers were seen in the B. rapa test sample, as they were in the F1-hybrids from controlled crosses and from the field. The marker frequencies of the test sample and F1-hybrids from field and controlled crosses are given in Table 1.
The assumption that the markers were selectively neutral seemed, however, to be true for only one of the three markers. The ISSR-2 marker present in 95% of the B. rapa test sample would be expected to be present in 48–95% of the F1-hybrids (the marker being heterozygous–homozygous in B. rapa). In the controlled cross, 53% of the F1-hybrids had the marker, and in the F1-hybrids from the field, 34 out of 59 or out of 1.25*59 (as 25% of the F1-hybrids are assumed to be undetected, Table 1), corresponding to 58% or 46%, should have the marker. Therefore, the frequencies of the ISSR-2 marker in the F1-hybrids from the controlled crosses and from the field corresponded well, and were within the expected range of the marker inherited from B. rapa to F1-hybrids. Contrary to this, the frequencies of the ISSR-1 and SSR-1 markers in the samples of F1-hybrids did not correspond to the expected inheritance from the marker frequencies in the B. rapa test sample. Based on these considerations, only progeny with the ISSR-2 marker were included in the data analysis.
The frequency of ISSR-2 in the F1-hybrids from the controlled crosses was used to estimate the total number of F1-hybrids in the field. Since 53% of the F1-hybrids in the controlled cross had the marker, 47% of the F1-hybrids from the field were undetected by our techniques. Therefore, the correction factor was n/53 × 100, with n=the number of identified F1-hybrids among the 50 progeny investigated per mother plant.
F1-hybrids per mother plant and per area
Out of the 50 progeny investigated, each mother plant produced 0–4 F1-hybrids at the 1:1 proportion, and 0–1 F1-hybrids at the 3:1 proportion. F1-hybrids were identified in 50–80% of the mother plants at the 1:1 proportion, and 10–20% of the mother plants at the 3:1 proportion.
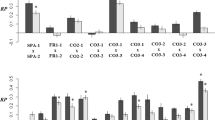
The frequency of F1-hybrids was significantly (P≤0.05) higher at the 1:1 proportion (2.3–5.3%) than the 3:1 proportion (0.4–0.8%) (Tables 2 and 3). Improved explanation of the data was not accomplished by other models. When the density was increased from 16 to 100 plants/m2, the frequency of F1-hybrids increased at the 1:1 proportion and decreased at the 3:1 proportion. The lowest frequency of F1-hybrids was obtained at intermediate and high density at the 3:1 proportion, and the highest frequency at the 1:1 proportion at high density.
From an agricultural viewpoint, the F1-hybrid production per area unit is interesting, because it takes into account plant density and seed number. The average number of F1-hybrids per square-meter is presented in Table 2. The average number of F1-hybrids per square-meter was significantly higher at the 1:1 proportion (Table 3). The density, the interaction between density and proportion and the covariates pod number, dry biomass and seed number did not improve the explanation of the data. When only analysing one factor at a time, a significant density effect on the number of F1-hybrids per square-meter at the 1:1 proportion and a significant effect of proportion on the number of F1-hybrids per square-meter at the intermediate density were revealed by t-tests (results not shown). The remaining t-tests were not significant.
Biomass components
Mean values of biomass components are presented in Table 4. Except for the 1000-kernel weight, which apparently is a conserved trait in oilseed rape, there was a significant decrease in the biomasses as the density was increased. The proportion between B. napus and B. rapa also affected the production of the biomass components, which was smaller at the 1:1 proportion compared with the 3:1 proportion. The difference was significant for the yield, the seed number and the total biomass (Table 5).
Discussion
Selection of markers for F1-hybrid evaluation
Normally, when using DNA markers as a diagnostic tool for the identification of specific genotypes, the markers are assumed to be inherited in Mendelian fashion. However, marker inheritance needs to be verified. In our experiment, different environmental conditions could have favoured different genes in the field and in the controlled crosses. A more likely explanation for the discrepancy between marker-frequencies in the three samples of plants could be that genetic drift was operating; the three samples were not equally large. Also, it could be that B. rapa individuals did not contribute equally to the pollination of the B. napus plants in the crosses and in the field.
The distribution of F1-hybrids among mother plants
Hybridisation can be very genotype-dependent (Pertl et al, 2002). Thus, to minimise the ‘genotype noise’, we chose as B. napus (♀) the variety Capitol, which should be homogenous because of its double-haploid origin. The homogeneity of Capitol had been confirmed in our previous study (Johannessen et al, 2002). The B. rapa population (♂) may, however, have been rather diverse with a high frequency of heterozygotes, as B. rapa is self-incompatible and the population was derived from a cross between four different populations. Since insects are the primary pollen vectors in oilseed rape (Hayter and Cresswell, 2003), adjacent B. rapa plants may have contributed the most to the pollination of each B. napus plant, and through this, made way for genotype-dependent differences in the hybridisation ability between fathers. However, no distinct deviations in F1-hybrid production were seen among the mother plants (0–4 F1-hybrids produced per mother plant).
Effects of density
The mean value of most biomass components per mother plant (Table 4) decreased significantly as density was increased. A great effect of plant density on the yield of individual plants of winter oilseed rape has previously been documented. Yield is most stable when plants are evenly distributed (Huhn, 1998; Diepenbrock, 2000). Our field experiment was set up very precisely, reducing differences in the intraplot density.
During 1996–2000, the average yield under Danish growth conditions was 40.7 hkg/ha for Capitol (Pedersen, 2000), giving a yield of 4.5–6.8 g per plant with densities of 60–90 plants/m2 as recommended. The plots that were most alike (intermediate to high density and 75% B. napus) performed rather well and within the same range.
The 1:1 proportion
When B. napus and B. rapa were equally represented (1:1 proportion), the frequency of F1-hybrids increased significantly as the density increased. Thus, intensified species interaction perhaps increased the pollination advantage of B. rapa. In a 1:1 species mixture with a plant density of 20 plants per square-meter, 9% F1-hybrids were produced on B. napus (spring type) (Jørgensen and Andersen, 1994). This 3–4 times higher percentage of F1-hybrids was found even though winter types of oilseed rape have a larger overlap in flowering time with B. rapa. However, Jørgensen and Andersen (1994) used more and different genotypes, and the environmental conditions were different, which may have improved the conditions for outcrossing.
Since the F1-hybrid production increased with reduced biomasses, the intensified competition perhaps affected both the vegetative fitness and the relative pollen contribution from B. napus and B. rapa. B. napus should have been expected to overcome a relative increase in B. rapa pollen since B. rapa pollen in B. napus (spring type) styles have significantly lower fitness than conspecific pollen (Hauser et al, 1997). In pure intraspecific pollinations, the pollen germination and growth takes 2 h in B. napus and 4 h in B. rapa to reach the ovaries (Röbbelen, 1960). Thus, as stated by Hauser et al (1997), it seems likely that B. rapa pollen in B. napus styles would be outcompeted by the conspecific pollen. Nevertheless, there may be a limit to the superiority of B. napus pollen. Most likely, the significant reduction in the reproductive fitness expressed by the seed number probably resulted from both reduced vegetative and reproductive fitness (ie fewer branches, fewer racemes, fewer flowers, less pollen).
The largest coefficients of variation (Table 4) were obtained at intermediate density. Studies made with soybean, turnip and radish showed no apparent correlation between the coefficient of variation and changes in density as reported by Kira et al (1953). They therefore deduced that intensified competition was quite uniformly shared among all plants, and the population as a whole reacted to the limited supply of growth factors. The coefficient was affected in our experiment and the difference may be ascribed to the presence of B. rapa and the effect of interspecific competition.
The 3:1 proportion
There was a nonsignificant density effect on the hybridisation frequency at the 3:1 proportion, despite a significant decrease in mean values of most biomass components per mother plant (Table 4). The effect of density on B. rapa and B. napus was probably equivalent, perhaps resulting in the same fitness ratio between B. napus and B. rapa.
The coefficient of variation increased with density, so the variation in biomass components increased even though the components on average became smaller. Thus, intensified competition resulted in a more diverse response among individual plants. In pure plots of soybean, turnip and radish, the effect of competition are shared uniformly among individuals (Kira et al, 1953). During free competition between plants, the effect is shared inconsistently, thus the process of free competition perhaps acted in the present study. If free competition was part of the increased variation, then, as suggested for the 1:1 proportion, this was maybe caused by the presence of B. rapa and the effect of interspecific competition.
The effect of proportion
The frequency of F1-hybrids was significantly lower at the 3:1 proportion (Tables 2 and 3), probably because B. rapa contributed less to the pollen being dispersed. Proportionality with the abundance of B. rapa would reduce the frequency of F1-hybrids per mother plant from the 1:1 proportion to the 3:1 proportion with 50%. This almost agreed at low density, but at intermediate and high density, the frequency was reduced by far more than 50%. Therefore, as the density was increased, the proportion became progressively more important.
Most biomass components per mother plant were significantly, or almost significantly larger at the 3:1 proportion. A spring type of B. napus likewise produced many more seeds in mixed plots with high frequency of itself (Pertl et al, 2002; Hauser et al, 2003).
The total biomass is regulated more precisely than the numerical size of a population and is thus expected to be the best measure of competition (Begon et al, 1990). B. napus was thus exposed to the most intense vegetative competition at the 1:1 proportion at high density when the frequency of F1-hybrids per mother plant was also highest, and the number of F1-hybrids per square-meter among the highest. The least intense competition was present at the 3:1 proportion at low density when the frequency of F1-hybrids and the number of F1-hybrids per square-meter were among the lowest. The total biomass was lower at the 1:1 proportion; B. rapa was therefore a stronger (vegetative) competitor than B. napus, affecting both the reproductive and vegetative fitness of B. napus; or to put it in another way, the weed decreased the crop output. This is in accordance with the expectations as weeds are characterised by their superior competitive traits.
Besides the vegetative competition, the composition of the pollen cloud probably affected the seed number, and, thus, the yield and total biomass. In pods containing both conspecific and heterospecific zygotes, the survival of B. napus (♀) × B. rapa zygotes was estimated to 15% compared with B. napus zygotes (Hauser et al, 1997). Therefore, reproductive interactions may partly explain why the seed number, yield and total biomass were lower at the 1:1 proportion and significantly different among proportions. The average number of seeds per pod was, however, only slightly lower at the 1:1 proportion, and not significantly different. Therefore, zygote death probably only explains a minor part of the differences between proportions.
The number of F1-hybrids per square-meter was significantly lower at the 3:1 proportion, which was consistent with the significantly lower frequency of F1-hybrids and seed number per mother plant.
The coefficient of variation was, in general, lower at the 3:1 proportion at low and intermediate densities, whereas it was opposite at high density. This means that both density and proportion affect competition, as expressed by variation in fitness parameters. The frequency of F1-hybrids and the number of F1-hybrids per square-meter was apparently not correlated to the size of the coefficients of variation.
Concluding remarks
In conclusion, the density effect was, for most of the fitness parameters, highly dependent on the proportion, whereas the converse was not the case (except for the trend in the coefficient of variation). The proportion of the two species was, therefore, apparently the more relevant environmental factor.
The frequency of F1-hybrids, the number of F1-hybrids per square-meter and the number of F1-hybrids per kg oilseed rape were lowest when B. napus was cultivated at intermediate to high densities, and the abundance of B. rapa in the field area was kept low. Therefore, cultivation of transplastomic oilseed rape varieties will probably produce least transplastomic F1-hybrids under these environmental conditions. Fortunately, this is in accordance with present good agricultural practice.
The present results are the first to report on spontaneous hybridisation between winter oilseed rape functioning as the mother plant and B. rapa as the pollen donor. From previous data on spring types of oilseed rape (Hauser et al, 1997), it is assumed that the ability to produce F1-hybrids in crosses between B. napus and B. rapa is lowest when B. napus functions as the mother. So, all in all, there is a clear benefit in using transplastomic lines for reducing transgene transfer to B. rapa.
References
Begon M, Harper JL, Townsend CR (eds) (1990). Intraspecific competition. In: Ecology: Individuals, Populations and Communities. Blackwell Scientific Publications Ltd: Massachusetts, Oxford, London, Edinburgh, Victoria. pp 197–239.
Charters YM, Robertson A, Wilkinson MJ, Ramsay G (1996). PCR analysis of oilseed rape cultivars (Brassica napus L. ssp. oleifera) using 5′-anchored simple sequence repeat (SSR) primers. Theor Appl Genet 92: 442–447.
Chèvre AM, Ammitzbøll H, Breckling B, Dietz-Pfeilstetter A, Frédérique E, Fargue A et al (2004). A review on interspecific gene flow from oilseed rape to wild relatives. In: Nijs H, Bartsch D, Sweet J (eds) Introgression from Genetically Modified Plants into Wild Relatives. CABI publishing: UK. pp 235–251.
Corriveau JL, Coleman AW (1988). Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am J Bot 75: 1443–1458.
Diepenbrock W (2000). Yield analysis of winter oilseed rape (Brassica napus L.): a review. Field Crop Res 67: 35–49.
Erickson L, Kemble R (1990). Paternal inheritance of mitochondria in rapeseed (Brassica napus). Mol Gen Genet 222: 135–139.
Hauser TP, Damgaard C, Jørgensen RB (2003). Frequency-dependent fitness of hybrids between oilseed rape (Brassica napus) and weedy B. rapa (Brassicaceae). Am J Bot 90: 571–578.
Hauser TP, Jørgensen RB, Østergård H (1997). Preferential exclusion of hybrids in mixed pollinations between oilseed rape (Brassicanapus) and weedy B. campestris (Brassicaceae). Am J Bot 84: 756–762.
Hayter KE, Cresswell JE (2003). An experimental evaluation of the relative importance of pollination by insects vs wind in oilseed rape (Brassica napus). In: Boelt B (ed) Proceedings of the 1st European conference on the co-existence of genetically modified crops with conventional and organic crops (GMCC-03), Snekkersten (DK), 13–14 November 2003. Danish Institute of Agricultural Sciences, Research Centre Flakkebjerg: Slagelse, Denmark. p 214.
Hou BK, Zhou YH, Wan LH, Zhang ZL, Shen GF, Chen ZH et al (2003). Chloroplast transformation in oilseed rape. Trans Res 12: 111–114.
Huhn M (1998). Ein allgemeiner Ansatz zur Quantifizierung des Einflusses der Güte der Sätechnik auf den Flächenertrag. J Agron Crop Sci 181: 249–255.
Johannessen MM, Mikkelsen TN, Jørgensen RB (2002). CO2 exploitation and genetic diversity in winter varieties of oilseed rape (Brassica napus); varieties of tomorrow. Euphytica 128: 75–86.
Jørgensen RB, Andersen B (1994). Spontaneous hybridization between oilseed rape (Brassica napus) and weedy B. campestris (Brassicaceae): a risk of growing genetically modified oilseed rape. Am J Bot 81: 1620–1626.
Khan MS, Maliga P (1999). Flourescent antibiotic resistance marker for tracking plastid transformation in higher plants. Nat Biotechnol 17: 910–914.
Kira T, Ogawa H, Sakazaki N (1953). Intraspecific competition among higher plants. I. Competition-yield-density interrelationship in regularly dispersed populations. J Institute Polytechnics Osaka City Univ 4: 1–16.
Landbo L, Andersen B, Jørgensen RB (1996). Natural hybridisation between oilseed rape and a wild relative: hybrids among seeds from weedy Brassica campestris. Hereditas 125: 89–91.
Landbo L, Jørgensen RB (1997). Seed germination in weedy Brassicacampestris and its hybrids with B. napus: Implications for risk assessment of transgenic oilseed rape. Euphytica 97: 209–216.
Milligan BG (1998). Total DNA isolation. In: Hoelzel AR (ed) Molecular Genetic Analysis of Populations: A Practical Approach. IRL Press: Oxford. pp 29–64.
Mogensen HL (1996). The hows and whys of cytoplasmic inheritance in seed plants. Am J Bot 83: 383–404.
Pedersen C (2000). Oversigt over Landsforsøgene 2000, Forsøg og undersøgelser i de landøkonomiske foreninger. Landbrugets rådgivningscenter, Landskontoret for planteavl: Skejby.
Pertl M, Hauser TP, Damgaard C, Jørgensen RB (2002). Male fitness of oilseed rape (Brassica napus), weedy B. rapa and their F1 hybrids when pollinating B. rapa seeds. Heredity 89: 212–218.
Röbbelen G (1960). Über die Kreuzungsunverträglichkeit verschiedener Brassica-Arten als Folge eines gehemmten Pollenschlauchwachstums. Züchter 30: 300–312.
Ruf S, Hermann M, Berger IJ, Carrer H, Bock R (2001). Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat Biotechnol 19: 870–875.
Sidorov VA, Kasten D, Pang SZ, Hajdukiewicz PTJ, Staub JM, Nehra NS (1999). Stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J 19: 209–216.
Skarjinskaia M, Svab Z, Maliga P (2003). Plastid transformation in Lesquerellafendleri, an oilseed Brassicacea. Trans Res 12: 115–122.
Svab Z, Hajdukiewicz PTJ, Maliga P (1990). Stable transformation of plastids in higher-plants. Proc Nat Acad Sci USA 87: 8526–8530.
Szewc-McFadden AK, Kresovich S, Bliek SM, Mitchell SE, McFerson JR (1996). Identification of polymorphic, conserved simple sequence repeats (SSRs) in cultivated Brassica species. Theor Appl Genet 93: 534–538.
Tolstrup K, Andersen SB, Boelt B, Buus M, Gylling M, Holm PB et al (2003). Report from the Danish working group on the co-existence of genetically modified crops with conventional and organic crops. DIAS Report Plant Production no. 94. Frederiksberg Bogtryk: Denmark.
Warwick SI, Simard MJ, Legere A, Beckie HJ, Braun L, Zhu B et al (2003). Hybridization between transgenic Brassicanapus L. and its wild relatives: Brassicarapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) OE Schulz. Theor Appl Genet 107: 528–539.
Acknowledgements
This work was made under the Center for Effects and Risks of Biotechnology in Agriculture (supported by the Danish Environmental Research Programme) and the Center for Bioethics and Risk Assessment (funded by the Danish Research Agency). We thank Carsten Lundsteen, The Abed Foundation, for procuring seeds of the oilseed rape variety Capitol. The staffs at Risø National Laboratory are thanked for their assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johannessen, M., Andersen, B. & Jørgensen, R. Competition affects gene flow from oilseed rape (♀) to Brassica rapa (♂). Heredity 96, 360–367 (2006). https://doi.org/10.1038/sj.hdy.6800796
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800796
Keywords
This article is cited by
-
Fitness of F1 hybrids between 10 maternal wild soybean populations and transgenic soybean
Transgenic Research (2021)
-
Pollen-mediated gene flow from transgenic cotton is constrained by physical isolation measures
Scientific Reports (2018)
-
Processes affecting genetic structure and conservation: a case study of wild and cultivated Brassica rapa
Genetic Resources and Crop Evolution (2009)
-
The variability of processes involved in transgene dispersal—case studies from Brassica and related genera
Environmental Science and Pollution Research (2009)
-
Competition Affects the Production of First Backcross Offspring on F1-hybrids, Brassica Napus × B. Rapa
Euphytica (2006)