Abstract
The genetic structure of populations of an arctic-montane herb, Saxifraga hirculus (Saxifragaceae), was analysed by means of chloroplast restriction fragment-length polymorphism. Sampled populations were distributed across Europe and North America (Alaska and Colorado). There was no evidence for geographically structured genetically divergent lineages, and although no haplotypes were shared between North America and Europe, the haplotypes from different continents were intermixed on a minimum spanning tree. European populations were much more highly differentiated and had much lower levels of haplotype diversity than their Alaskan counterparts. Centres of haplotype diversity were concentrated in those Alaskan populations located outside the limits of the last (Wisconsin) glaciation, suggesting that they may have acted as refugia during the Pleistocene. It was not possible to identify putative migration routes or corresponding refugia in the European genepool. One British population, from the Pentland Hills, was genetically very distant from all the others, for reasons that are as yet unknown.
Similar content being viewed by others
Introduction
Several recent studies have examined the patterns of genetic variation in plant species with arctic distributions (Tremblay and Schoen, 1999; Abbott et al, 2000; Abbott and Brochmann, 2003; Abbott and Comes, 2003). The aim has been to produce evidence bearing on the hypothesised existence of northern refugia, areas in which species may have survived the last glaciation in situ. Particular attention has been directed at the Nordic region, where the debate over glacial survival versus the alternative tabula rasa hypothesis has been particularly fierce (Berg, 1963; Borgen, 1987; Dahl, 1987; Nordal, 1987; Birks HJB, 1993; Birks HH, 1994). Patterns of genetic variation in this area in a range of species, including the outbreeding Saxifraga oppositifolia (Gabrielsen et al, 1997) and the inbreeding Saxifraga cespitosa (Tollefsrud et al, 1998), show very little indication of geographical race formation or of deep geographical structure (Brochmann et al, 1996). Rather, studies have concluded that levels of gene flow have been so high that any evidence of centres of genetic diversity, such as might be expected to characterise refugia, is likely to have been obliterated. Thus glacial refugia, if they existed in the Nordic region, are irrelevant to present day patterns of variation (Brochmann et al, 1996). On the other hand, at a wider spatial scale, a survey of chloroplast DNA restriction fragment-length polymorphism (RFLP) in S. oppositifolia across the whole of the arctic did reveal significant phylogeographic structure and the presence of two principal lineages, one with a predominantly North American distribution and one with a predominantly Eurasian distribution (Abbott and Brochmann, 2003). A high level of chloroplast diversity was detected in Alaska, contributing to the growing body of evidence that Beringia may have acted as a refugium for many arctic plants throughout the ice ages (Hultén, 1937; Abbott and Brochmann, 2003). In this context, the aim of the present work is to investigate the population genetic structure of another arctic-montane Saxifraga species, Saxifraga hirculus L., to assess the extent to which similar phylogeographic patterns occur in arctic plant species. Specifically, we aim to (a) investigate whether there is evidence for geographically structured genetically divergent lineages in North America versus Europe, and (b) establish how levels of genetic diversity correlate with present day abundance and past glacial history. The approach taken has been to study RFLP variation in chloroplast DNA, which is inherited maternally in the Saxifragaceae (Soltis et al, 1990).
Study species
S. hirculus is a loosely tufted, rhizomatous, perennial herb with a circumpolar, arctic-montane distribution (Hultén, 1971). Its main distribution in the arctic comprises Alaska, northern Canada, Greenland, Iceland, Svalbard, Scandinavia, and northern Russia through to Beringia. Its distribution extends southwards disjunctly to the Rocky Mts of Colorado, the Caucasus, Central Asia and the outer ranges of the Himalaya. In Europe the species occurs discontinuously southwards to Switzerland and central Romania. S. hirculus is abundant in arctic regions where it occurs mainly in water-saturated moss-tundra; in more southern localities in Europe it typically inhabits base-rich flushes and mires. During the 19th and 20th centuries, populations in the southern part of its European range suffered a serious decline, attributable to habitat degradation and fragmentation with afforestation, drainage and overgrazing being important contributing factors (UK Biodiversity Group, 1995). The species is now extinct in Austria, the Czech republic and the Netherlands, and is severely depleted in southern Sweden, Germany, the Alps, Poland and the Baltic states, where it is registered as endangered or vulnerable (Jalas et al, 1999). It has also declined in the British Isles, where it is now restricted to very localised patches in the northern Pennines, the Pentland Hills, the Grampians and north-eastern Scotland and a few localities in northern and western Ireland (Preston et al, 2002). It is a priority species listed in Annexes II and IV of the EU Habitats and Species Directive, and there are plans for reintroductions as part of the UK BAP for the species (UK Biodiversity Group, 1995; Welch, 2002).
Owing to concerns regarding its conservation there has been renewed interest in the reproductive biology of S. hirculus, and several studies have reported on pollination (Olesen and Warncke, 1989a, 1989b, 1990; Warncke et al, 1993), seed set (Ohlson, 1988; Dahlgaard and Warncke, 1995) and population differentiation (Ohlson, 1986, 1989a; Olesen and Warncke, 1990). The flowers are strongly protandrous but self-compatible (Olesen and Warncke, 1989a). They are visited by a large spectrum of insects but mainly by syrphid flies, with different insect species being important in different regions (Warncke et al, 1993). The seeds of S. hirculus have no special adaptations for dispersal but are dropped near the parent plant when shaken from the capsules by wind or rain, the average dispersal distance by this method being 13 cm (Olesen and Warncke, 1989b). It has also been speculated, however, that large herbivores, such as deer, might move seeds from place to place (Olesen and Warncke, 1990). Seedling recruitment appears to depend on the availability of suitable bare ground (Ohlson, 1989b).
Morphologically and cytologically the species is polymorphic, and varying numbers of intra-specific taxa have been recognised (Engler and Irmscher, 1916/19; Hedberg, 1992), although the distinctions are often blurred and intermediates occur. The morphological variation appears to be correlated to some extent with differences in chromosome number, such that diploids (2n=16) and tetraploids (2n=32) are often associated with particular phenotypes. Occasional triploids also occur. Based on the most recent taxonomic treatment of the complex (Hedberg, 1992), an imperfect distinction can be made between largely circumboreal-montane populations, in which the flowering stems are tall, bear many leaves and at least two flowers, and whose sepals are reflexed at anthesis (ssp. hirculus, 2n=32) versus predominantly circumpolar, arctic populations whose flowering stems are shorter, bear fewer leaves and solitary flowers with sepals erect or spreading at anthesis. The arctic populations can be divided into those primarily from the palaearctic, in which the base of the petals is auriculate to truncate with a short claw (ssp. compacta O. Hedb., 2n=32) and those from the nearctic, in which the petal bases tend to be cuneate and lack a clearly defined claw (ssp. propinqua (R. Br.) Löve and Löve, 2n=16). Alaskan material is particularly variable and contains all three of these subspecies, as well as numerous intermediates (Hultén, 1968, pp 568; Hedberg, 1992). The outlying populations from Colorado (ssp. coloradensis O. Hedb., 2n=16) differ from ssp. hirculus not only in their diploid status but also in having solitary flowers. At least some of the morphological variation seen in the species as a whole appears to be environmentally induced (Ohlson, 1989a, 1989b), not least the number of flowers produced per stem (Hedberg, 1992).
Materials and methods
Materials
Leaf samples from a total of 487 individuals were collected (at a minimum interval of 2 m between sampled plants) from 31 populations from Europe (England, Scotland, Ireland, Iceland, Svalbard, Denmark, Switzerland) and North America (Alaska, Colorado). Full details are provided in Table 1. Voucher specimens have been deposited in E (herbarium, Royal Botanic Garden, Edinburgh). The sampling strategy was designed to include populations from areas that were heavily glaciated during the last ice age (Europe, Colorado and southern Alaska), and those from areas that are considered to have remained ice free (central Alaska). The sampling also included populations from areas where the species is now abundant (Alaska, Iceland, Svalbard) and from where it is now rare (England, Scotland, Ireland, Denmark, Switzerland and Colorado).
Molecular methods
DNA extractions were carried out using the CTAB method of Doyle and Doyle (1990). A total of 17 regions of chloroplast DNA were examined: atpB-rbcL, trnL-F, trnC-trnD, trnF-trnVr, trnD-trnT, trnS-trnG, trnK2-trnQr, trnQ-trnRr, trnK2-trnK1, trnH-trnK, trnT-psbCr, rpoC1-trnCr, trnH-psbA, psbAr-trnFm, rpl20-rps12, psbB-psbF and trnV-rbcLr. These regions were PCR amplified using universal primers designed by Taberlet et al (1991), Demesure et al (1995), Dumolin-Lapegue et al (1997), Chiang et al (1998) and Hamilton (1999). The products were cut with individual restriction enzymes: AluI, DraI, HhaI, HincII, HinfI, MseI, MspI, RsaI and TruI. Choice of enzyme was partly arbitrary and partly based on analysis of sequences of the regions atpB-rbcL, rpl20-rps12, trnH-psbA, trnL-F, and trnS-trnG, from a subset of the samples. When sequencing revealed polymorphisms, restriction enzyme assays were designed (where possible) using the program Webcutter (http://www.firstmarket.com/cgi-bin/cutter) and extended to the full set of samples.
Restriction enzyme digests were carried out following the manufacturer's (GibcoBRL) instructions on 10 μl of PCR product, to which 0.2 μl of enzyme, 0.2 μl of BSA, 2 μl of 10 × buffer and 7.6 μl H2O, was added. They were incubated at 37°C in a water bath for 2 h. The products of the digests were separated either on agarose or polyacrylamide gels and visualised using ethidium bromide and ultra violet light. In many instances, particularly where intrapopulation variation occurred, duplicate DNA extractions were carried out and the haplotypes confirmed. In order to avoid scoring the same mutation twice, two polymorphisms from the same region were used only if they distinguished different haplotypes.
A small inversion detected in the region trnT-psbA and several point mutations present outside restriction sites in other sequenced regions were screened in one individual of each haplotype from each population of the European samples.
Data analysis
Variation within populations was estimated by dividing the number of haplotypes recovered by the number of individuals assayed (=haplotype diversity) and also by calculating the gene diversity (HE) using GDA (Lewis and Zaykin, 2000). Relationships between all chloroplast haplotypes were estimated by means of a minimum spanning network, constructed using Arlequin (Schneider et al, 2000).
A hierarchical analysis of molecular variance (AMOVA) was also undertaken using Arlequin to assess the amount of variation among regions (Europe and North America). Levels of population differentiation were measured by estimating GST, firstly by taking the data as a whole, and then with European and North American populations treated separately (calculated using PERMUT: http://www.pierroton.inra.fr/genetics/labo/Software/Permut/). To establish the extent to which mutational differences between haplotypes contributes towards population differentiation, the program PERMUT was used to estimate NST, again combining and then separating the North American and European data as before. The significance of the difference between GST and NST was tested via permutation tests.
Results
Of the 17 regions of chloroplast DNA examined, seven contained usable polymorphisms: atpB-rbcL, trnC-trnD, trnD-trnT, trnF-trnVr, trnK2-trnQ, trnL-F and trnS-trnG, which were scored in all 487 individuals. These polymorphisms served to distinguish 40 haplotypes.
Haplotype relationships
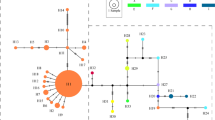
A minimum spanning network illustrating the relationships between the 40 haplotypes is shown in Figure 1. It can be seen that most differ by only a single mutational step from one another. There are, however, a few which are more isolated, including most notably Haplotype 1 from the Pentland Hills in Scotland, which resides at the end of a long branch, separated by five unique mutations from Haplotype 2 from the Grampians. Inclusion of sequence data (not shown) increases this mutational distance to six steps. The European haplotypes occupy scattered positions relative to their North American counterparts.
Minimum spanning network for European and North American chloroplast haplotypes of Saxifraga hirculus. Numbers represent haplotypes with their regions of origin denoted by symbols (see key, shaded=Europe, open=N. America). Superimposed symbols indicate the co-occurrence of haplotypes in a region. Small circles denote hypothetical intermediate haplotypes, each differing by one mutational step.
Haplotype geography
No haplotypes were shared between Europe and North America. Seven haplotypes were recovered from European populations (Table 2). Of these, three were found in more than one population. Haplotype 3 is the most widespread, being shared among all populations from Iceland, quadrats 1 to 4 from Rosborg in Denmark, all plants from Northern Ireland, and two of the four populations from the English Pennines. Haplotype 2 is shared by the three populations from the Scottish Grampians and two of the four populations from the English Pennines. The plants from Switzerland and plants from quadrats 5 to 7 from Rosborg in Denmark share Haplotype 6. Four European haplotypes were private (restricted to a single population). Thus, Haplotype 1 is present only in populations in the Pentlands in Scotland. Haplotypes 4 and 5 are present in 20 and two individuals, respectively, from Svalbard. Haplotype 7 is present in only one individual from Iceland.
In all 33 haplotypes were recovered from North American populations (Table 3; Figures 2a and b). Haplotypes 8–38 occur in Alaska and numbers 39 and 40 are from Colorado. Six haplotypes were found in more than one population (Table 3). Thus, Haplotype 13 is the commonest haplotype in Alaska, present in 42 individuals among eight populations, occurring in Denali National Park, along the Richardson Highway (Fielding Lake) and in the Nome area. Haplotype 17 is almost as widespread, being present in 15 individuals from five populations in the same three areas. Haplotype 22 is also common, present in 28 individuals in five populations: Primrose Ridge in Denali, and four populations from the Nome area. Haplotypes 15 and 16 are present in both the Denali and Fielding Lake populations. Haplotype 30 is present in both the Penny River and the Kigluaik Mountains populations from the Nome area. Most (27/33) haplotypes, however, are private, that is, restricted to a single population (Table 3). Haplotype 8 is present in the Summit Lake population only. Haplotypes 9–12 and 14 are unique to the Sable Pass population in Denali National Park. Haplotypes 18–21 are all unique to Highway pass in Denali. Haplotype 23 is present only in the Primrose Ridge population in Denali. Haplotypes 24–26 are present only in the Fielding Lake population. Haplotype 27 is present only in the Kougarok Road population, north of Nome. Haplotypes 28 and 29 are present only in the Penny River population from the Nome area, and Haplotype 31 is present only in the Kigluaik Mountains population. Haplotypes 32–37 are present only in the Teller population. Haplotype 38 is present only in the mile 56 population from the Nome area. Haplotypes 39 and 40 are from the Summit Lake and the North Tarryall Peak populations, respectively, in Colorado.
Genepool structure and population differentiation
The much lower haplotype diversity in Europe compared with North America is summarised in Table 4. Here it can be seen that in Europe fewer than 18% of the populations are polymorphic and 57% of the haplotypes are private, whereas in North America the respective figures are 71 and 82%. Likewise, the regional level gene diversity and number of haplotypes revealed are HE=0.66, seven haplotypes in Europe and HE=0.90, 33 haplotypes in North America. In Europe, Britain has the highest haplotype diversity. In North America the highest diversity is found in those Alaskan populations situated in or on the edge of the areas that escaped the last (Wisconsin) glaciation. They contrast markedly with the three populations from areas that were not ice free, viz. Summit Lake and the two from Colorado, which are genetically uniform (Figure 2).
A hierarchical AMOVA analysis with populations grouped into regions (North America versus Europe) indicated that 10.8% of variation was partitioned among regions, 65.9% of variation was among populations within regions, and the remainder (23.3%) of variation was within populations (all partitions were significant).
Estimates of differentiation (GST, NST) among populations from Europe, North America and over the total range are shown in Table 5. Such estimates are often affected by the geographical span of the study. In the present case, however, the respective sample distances of the European and North American populations are similar. Comparison between the much higher levels of differentiation in Europe (GST=0.930) versus North America (GST=0.489) is therefore legitimate.
Comparisons between values of GST (which does not take the number of mutational differences between haplotypes into account) and NST (which does) show that over all populations, the NST value of 0.779 and GST value of 0.731 are not significantly different (Table 5). Likewise, although NST was slightly higher than GST in analyses just involving European and just Alaskan populations, these differences were not significant. In the comparison including all North American populations, the difference was just significant (P<0.05 but >0.01).
Discussion
Phylogeographic lineages
Although no chloroplast haplotypes are shared between European and North American populations of S. hirculus, there is no molecular evidence for two distinct geographically structured lineages as in the case of S. oppositifolia (Abbott et al, 2000). This illustrates the complex nature and variation in glacial/interglacial phylogeography (Abbott and Brochmann, 2003) and the inherent difficulties in finding common patterns. Overall, in S. hirculus, there is little relationship between the geographical origin and the genetic similarity of the chloroplast haplotypes, indicating that the haplotypes arose before they dispersed to their present day locations. European haplotypes are scattered on the haplotype tree and are almost as likely to be more similar to an Alaskan haplotype as they are to one from Europe. And when two European haplotypes occur in the same population, such as in Denmark and in Svalbard, they are not genetically the most similar. This pattern is characteristic of old lineages that have become geographically dispersed since the time they arose by mutation (Pons and Petit, 1996). Such widespread dispersal should be seen in the context of the availability of abundant open habitat following Pleistocene glaciations. In contrast, the single individual in Iceland with a different haplotype differs from the common Icelandic haplotype (Haplotype 3) by only one mutation (Figure 1), and this haplotype could have evolved more recently, in situ.
The most divergent haplotype in the data set is restricted to a single population in southern Scotland in the Pentland Hills. The genetic distinctness of this otherwise unremarkable population is surprising. It is unlikely to be a case of local chloroplast capture from another species since S. hirculus is the only member of section Ciliatae subsection Hirculoides with a distribution outside of the high mountains of central Asia and the Sino-Himalaya region. Given that southern Scotland was covered by ice 10 000 years bp, either a local refugium or dispersal from some as yet unsampled population are two possible explanations. Greater understanding may be obtained with improved sampling in northern Europe, particularly in the Eurasian arctic and the high mountains of central Asia, such as the Altai, to which S. hirculus extends. Such sampling might reveal matching or linking haplotypes. It is also true that many European populations have died out, possibly causing intermediate haplotypes to be lost and thereby obscuring relationships between present day populations.
Partitioning of haplotype diversity
Overall, the differentiation in chloroplast types among populations is high, with >70% of haplotype variation being distributed between populations. This conforms to expectations for a small herb that produces seeds without a specialized dispersal mechanism. This pattern is much more marked in Europe than it is in North America (GST 0.930 and 0.489, respectively). In Europe, many populations show fixed differences in chloroplast types and in the UK for instance, populations in the Pennines, as close as 8 km, are fixed for different chloroplast haplotypes. This indicates that no seed dispersal and establishment is occurring over distances as short as 8 km. In Denmark the situation appears to be even more marked. Here individuals from quadrats 1 to 4 contain one haplotype (Haplotype 3), whereas those from quadrats 5 to 7 contain another (Haplotype 6). The two sets of quadrats are separated from each other by only 9–45 m. Seed dispersal and establishment is thus perhaps even more restricted than would have been deduced from the Pennine populations, and these data, coupled with field observation (eg Olesen and Warncke, 1989b), suggest there is little opportunity for gene flow and dispersal among the patchy habitat fragments that mark the discontinuous distribution of this species in its more southerly European localities.
There is thus something of a paradox regarding the lack of geographical structure in chloroplast lineages at regional geographical scales (Europe and North America), and the local differentiation of populations in Europe. The former suggests efficient seed dispersal, the latter suggests the opposite. One possible explanation for this discrepancy is that these data reflect different points in history. The lack of regional reciprocal monophyly can be attributed to the likely availability of open habitat following the Pleistocene glaciations offering an ideal environment for reproduction, dispersal and establishment. However, in more recent times and at more local scales, particularly at the southern edge of the range, the rarity of S. hirculus and its patchy fragmented distribution are likely to have led to reduced opportunities for dispersal and hence increased local population differentiation. This fragmentation is likely to have been exacerbated at some sites by grazing (eg in the UK), and close cropping of the flowers by sheep may serve to further reduce opportunities for gene flow.
Amounts of haplotype diversity
The North American genepool contains much more chloroplast haplotype variation than its European counterpart. The high levels of diversity are not a sampling artefact: our within-population sampling strategy was deliberately and unusually intensive, and designed to test rigorously hypotheses about relative levels of haplotype diversity. In particular, we are confident that the uniformity or near-uniformity of populations in Britain, Denmark and Iceland is a real contrast to the extraordinary diversity seen in some North American populations. The higher diversity of the North American genepool compared with that in Europe is reflected overall in terms of haplotype richness (33 versus 7), in the proportion of haplotypes distinguishable (0.163 versus 0.025), and in the proportion of populations that are polymorphic (71% versus 18%). Particularly diverse are those Alaskan populations located in or on the margin of areas unglaciated during the last (Wisconsin) glaciation (Figure 2b). Thus, the six populations from the Nome area, the four from the Denali area, and the one from Fielding Lake off the Richardson Highway, all display high levels of haplotype diversity (Table 3, Figure 2b). They contrast markedly with the uniformity of the populations from Summit Lake and Colorado, all of which are located in places covered by ice during the last glaciation. The high cpDNA diversity of S. hirculus in populations from unglaciated Alaska indicates that they may have been large for a long period of time, and supports theories that the Beringian region acted as a glacial refugium for Arctic plants during the ice ages (Hultén, 1937; Abbott and Brochmann, 2003). The chloroplast diversity parallels to some extent morphological diversity in the species: three of the four subspecies of S. hirculus have been recorded from Alaska. Recently, molecular evidence has been presented that Dryas integrifolia (Tremblay and Schoen, 1999), and S. oppositifolia (Abbott et al, 2000) survived the Quaternary glaciations in this region, and a hotspot of cpDNA diversity has also been recorded here for Saxifraga cernua (Brochmann et al, 2003). Fossil evidence shows that Beringia was covered by various tundra types in this period (Ritchie and Cwynar, 1982; Edwards et al, 2000), and macrofossils of S. oppositifolia have been found on the northern part of the Seward Peninsula of Alaska during the last full glacial (Goetcheus and Birks, 2001). There are thus several strands of evidence that support the importance of Alaska as a refugium for arctic plants. However, one qualifier is that our lack of sampling in Asia and northern/eastern North America does not allow us to evaluate the extent to which some of the cpDNA diversity in S. hirculus in Alaska is attributable to immigration from elsewhere (cf. Petit et al, 2003).
The low level of chloroplast diversity in Europe, where all sampled populations are from countries that were recently glaciated, is largely uncoupled from local population sizes and abundance. S. hirculus is common and widespread in Iceland, yet only two haplotypes were found from 85 samples from across the country, with one of these present in only one plant. Similar (or higher) levels of diversity are found in other European countries where the species is much rarer, such as the UK where it is restricted to extremely localised patches in a heavily grazed landscape. This illustrates the importance of population history over contemporary population sizes as a major determinant of patterns of population genetic variation. Presumably the large areas of tundra in continental Europe would have provided a suitable habitat for this species during glacial maxima. It can be hypothesised that this diversity has been eroded during bottlenecks associated with postglacial colonisation, and any remaining within-population diversity further lost due to recent habitat fragmentation in the more southerly European populations.
Taxonomic implications
The molecular data lend little support to the morphological recognition of four subspecies of S. hirculus in the geographical area studied here. Thus, in Europe, material from Iceland and Svalbard is recognised as ssp. compacta, distinguished by its short, one-flowered stems from ssp. hirculus (taller, at least two-flowered stems) from localities to the south. This distinction is not reflected by the chloroplast haplotype data, where Iceland at least is dominated by a haplotype that occurs also in England, N. Ireland and Denmark. Similarly it is impossible to make a distinction on haplotype evidence between the North American ssp. propinqua and the primarily European ssp. hirculus, so intermixed are they in the minimum spanning network (Figure 1). It should be admitted, however, that comparison here is confounded by the occurrence in Alaska of specimens that are identifiable as ssp. hirculus and the fact that the morphology of specimens from which the Alaskan chloroplast haplotypes were taken is unknown. In light of all the above, it is doubtful, therefore, whether much importance should be attached to the finding that the Colorado populations (ssp. coloradensis) cluster with other North American material but not with each other in this network (Figure 1).
References
Abbott RJ, Brochmann C (2003). History and evolution of the arctic flora: in the footsteps of Eric Hultén. Mol Ecol 12: 299–313.
Abbott RJ, Comes HP (2003). Evolution in the Arctic: a phylogeographic analysis of the circumarctic plant, Saxifraga oppositifolia (Purple saxifrage). New Phytologist 161: 211–224.
Abbott RJ, Smith LC, Milne RI, Crawford RMM, Wolff K, Balfour J (2000). Molecular analysis of plant migration and refugia in the Arctic. Science 289: 1343–1346.
Berg RY (1963). Disjunksjoner i Norges fjellflora og de teorier som er framsatt til forklaring av dem. Blyttia 21: 133–177 (in Norwegian with English summary).
Birks HH (1994). Plant macrofossils and the nunatak theory of per-glacial survival. In: Lotter AF, Ammann B (eds) Dissertationes Botanicae; A festschrift for Gerhard Lang: Contributions to the systematics and evolution, floristics and geobotany. J. Cramer: Berlin. pp 129–143.
Birks HJB (1993). Is the hypothesis of survival on glacial nunataks necessary to explain the present-day distributions of Norwegian mountain plants. Phytocoenologia 23: 399–426.
Borgen L (1987). Postglacial evolution in the Nordic flora a review. Blyttia 45: 147–168.
Brochmann C, Gabrielsen TM, Hagen A, Tollefsrud MM (1996). Seed dispersal and molecular phylogeography: glacial survival, tabula rasa, or does it really matter? Det Norske Videnskaps-Akademi. I Mat Nat Kl Avh Ny Serie 18: 54–68.
Brochmann C, Gabrielsen TM, Nordal I, Landvik JY, Elven R (2003). Glacial survival or tabula rasa? The history of the North Atlantic biota revisited. Taxon 52: 417–450.
Chiang T-Y, Schaal BA, Peng C-I (1998). Universal primers for amplification and sequencing a noncoding spacer between atpB and rbcL genes of chloroplast DNA. Botanical Bull Acad Sinica 39: 245–250.
Dahl E (1987). Alpine-subalpine plant communities of south Scandinavia. Phytocoenologia 15: 455–484.
Dahlgaard J, Warncke E (1995). Seed set and germination in crosses within and between two geographically isolated small population of Saxifraga hirculus in Denmark. Nordic J Bot 15: 337–341.
Demesure B, Sodzi N, Petit RJ (1995). A set of universal primers for amplification of polymorphic non-coding regions of mitochondrial and chloroplast DNA in plants. Mol Ecol 4: 129–131.
Doyle JJ, Doyle JL (1990). Isolation of plant DNA from fresh tissue. Focus 12: 13–15.
Dumolin-Lapegue S, Pemonge M-H, Petit RJ (1997). An enlarged set of consensus primers for the study of organelle DNA in plants. Mol Ecol 6: 393–397.
Edwards ME, Anderson PM, Brubaker LB, Ager TA, Andreev AA, Bigelow NH et al (2000). Pollen-based biomes for Beringia 18,000, 6000 and 0 14C yr BP. J Biogeog 27: 521–555.
Engler A, Irmscher E (1916/19). Saxifragaceae – Saxifraga I. Das Pflanzenreich 1, IV, 117, 1. W. Engelman: Leipzig.
Gabrielsen TM, Bachmann K, Jakobsen KS, Brochmann C (1997). Glacial survival does not matter: RAPD phylogeography of Nordic Saxifraga oppositifolia. Mol Ecol 6: 831–842.
Goetcheus VG, Birks HH (2001). Full-Glacial upland tundra vegetation preserved under tephra in the Beringia National Park, Seward Penninsula, Alaska. Quat Sci Rev 20: 135–147.
Hamilton MB (1999). Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Mol Ecol 8: 513–525.
Hedberg KO (1992). Taxonomic differentiation in Saxifraga hirculus L. (Saxifragaceae) – a circumpolar Arctic-Boreal species of Central Asiatic origin. Bot J Linn Soc 109: 377–393.
Hultén E (1937). Outline of the History of Arctic and Boreal Biota during the Quarternary Period. Lehre J Cramer: New York.
Hultén E (1968). Flora of Alaska and Neighbouring Territories. Stanford University Press: California.
Hultén E (1971). The circumpolar plants. II. Dicotyledons. K Sven Vetenskapsakad Handl, Fjärde Serien 13: 1–463.
Jalas J, Suominen J, Lampienin R, Kurtto A (1999). Atlas Florae Europaeae, Distribution of Vascular Plants in Europe 12; Resedaceae to Platinaceae. The Committee for Mapping the Flora of Europe and Societus Biologica Fennica Vanamo: Helsinki.
Lewis PO, Zaykin D (2000). GDA 1 d15 http://hydrodictyon.eeb.uconn.edu/people/plewis/software.php
Nordal I (1987). Tabula rasa after all? Botanical evidence for ice free refugia in Scandinavia reviewed. J Biogeogr 14: 377–388.
Ohlson M (1986). Reproductive differentiation in a Saxifraga hirculus population along an environmental gradient on a central Swedish mire. Holarctic Ecol 9: 205–213.
Ohlson M (1988). Size-dependent reproductive effort in three populations of Saxifraga hirculus in Sweden. J Ecol 76: 1007–1016.
Ohlson M (1989a). Ecotypic differentiation and phenotypic plasticity in Saxifraga hirculus populations in central and northern Sweden. Holarctic Ecol 12: 46–53.
Ohlson M (1989b). Dynamik I nord-och mellansvenska populationer av myrbräcka. Svensk bot Tidskr 83: 1–11.
Olesen JM, Warncke E (1989a). Temporal changes in pollen flow and neighbourhood structure in a population of Saxifraga hirculus L. Oecologia 79: 205–211.
Olesen JM, Warncke E (1989b). Flowering and seasonal changes in flower sex ratio and frequency of flower visitors in a population of Saxifraga hirculus. Holarctic Ecol 12: 21–30.
Olesen JM, Warncke E (1990). Morphological, phenological and biochemical differentiation in relation to gene flow in a population of Saxifraga hirculus. Sommerfeltia 11: 159–172.
Petit RJ, Aguinagalde I, de Beaulieu JL, Bittkau C, Brewer S, Cheddadi R et al (2003). Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300: 1563–1565.
Pons O, Petit RJ (1996). Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144: 1237–1245.
Preston CD, Pearman DA, Dines TD (2002). New Atlas of the British and Irish Flora. Oxford University Press: Oxford.
Ritchie JC, Cwynar LC (1982). The late-quaternary vegetation of the North Yukon. In: Hopkins DM, Matthews JV, Schweger CE, Young SB (eds) Paleoecology of Beringia. Academic Press: New York pp. 113–126.
Schneider S, Roessli D, Excoffier L (2000). Arlequin ver. 2.000: a software for population genetics data analysis. Available from: http://lgb.unige.ch/arlequin/.
Soltis DE, Soltis PS, Ness BD (1990). Maternal inheritance of the chloroplast genome in Heuchera and Tolmiea (Saxifragaceae). J Hered 81: 168–170.
Taberlet P, Gielly L, Pautou G, Bouvet J (1991). Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molec Biol 17: 1105–1109.
Tollefsrud MM, Bachmann K, Jakobsen KS, Brochmann C (1998). Glacial survival does not matter – II: RAPD phylogeography of Nordic Saxifraga cespitosa. Mol Ecol 7: 1217–1232.
Tremblay NO, Schoen DJ (1999). Molecular phylogeography of Dryas integrifolia: glacial refugia and postglacial recolonization. Mol Ecol 8: 1187–1198.
UK Biodiversity Group (1995). Biodiversity: the UK Steering Group Report, Volume 2: Action Plans. HMSO: London.
Warncke E, Terndrup U, Michelsen V, Erhardt A (1993). Flower visitors to Saxifraga hirculus in Switzerland and Denmark, a comparative study. Bot Helvetica 103: 141–147.
Welch D (2002). The establishment of recovery sites for Saxifraga hirculus in NE Scotland. Bot J Scotland 54: 75–88.
Acknowledgements
We are grateful to Tove Gabrielsen, Keith Watson, Mark Wright, Jane Squirrell, David Welsh, Herve Freymond, and the late Peter Kelly for collecting plant material, to SNH and EN for supporting investigations on UK populations, to the Botanical Society of the British Isles for supporting our field work in Iceland, to the Louise Home award to CO for supporting field work in Colorado, and to two anonymous referees for comments on the paper. The Royal Botanic Garden Edinburgh is funded by the Scottish Executive Environmental and Rural Affairs Department.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliver, C., Hollingsworth, P. & Gornall, R. Chloroplast DNA phylogeography of the arctic-montane species Saxifraga hirculus (Saxifragaceae). Heredity 96, 222–231 (2006). https://doi.org/10.1038/sj.hdy.6800785
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800785
Keywords
This article is cited by
-
Phylogeography and ecological niche modeling implicate multiple microrefugia of Swertia tetraptera during quaternary glaciations
BMC Plant Biology (2023)
-
Biogeography of North Pacific Isoëtes (Isoëtaceae) inferred from nuclear and chloroplast DNA sequence data
Journal of Plant Biology (2016)
-
Chloroplast-specific universal primers and their uses in plant studies
Biologia plantarum (2011)
-
The evolutionary history of the Arabidopsis lyrata complex: a hybrid in the amphi-Beringian area closes a large distribution gap and builds up a genetic barrier
BMC Evolutionary Biology (2010)