Abstract
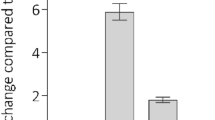
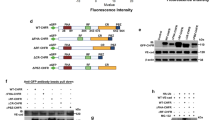
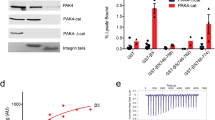
We explored the possibility of using a genetic approach to inhibit integrin-mediated endothelial cell adhesion and survival. We constructed recombinant adenoviruses (Ads) expressing chimeric proteins consisting of the cytoplasmic and transmembrane domains of integrin β1 (CH1), β3 (CH3) or the β1 transmembrane domain alone (CH2) connected to the extracellular domain of L3T4 placed under the control of the CMV promoter (AdCMV) or the endothelial cell specific Tie-1 promoter (AdTie). All constructs were expressed in a dose- and time-dependent manner with over 90% of cells expressing the constructs within 24 h (AdCMVs) or 72 h (AdTies) after infection. Confluent monolayers of HUVEC infected with AdCMVCH1 or AdCMVCH3 detached from the substrate in a time- and dose-dependent manner with over 95% of the cells being detached 2 days (AdCMVs) or 3 to 4 days (AdTies) after infection. Cell detachment was preceded by the disruption of focal adhesions and reorganization of the actin cytoskeleton and was associated with a reduced ligand-binding activity of β1, while cell surface density of β1 integrins remained unchanged. Detached cells failed to re-adhere to different matrix proteins, without, however, any specificity toward β1 or β3 integrin-mediated adhesion. Upon detachment, HUVEC rapidly died by apoptosis. These results demonstrate that dominant negative inhibition of integrin function is an effective approach to disrupt endothelial cell adhesion and survival in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Folkman J . Angiogenesis in cancer, vascular, rheumatoid and other disease Nature Med 1995 1: 27–31
Boehm T, Folkman J, Browder T, Oreilly MS . Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance Nature 1997 390: 404–407
Bergers G et al. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice Science 1999 284: 808–812
Parangi S et al. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth Proc Natl Acad Sci USA 1996 93: 2002–2007
Stromblad S, Cheresh DA . Cell adhesion and angiogenesis TIBS 1996 6: 462–468
Hynes O . Integrins: versatility, modulation, and signaling in cell adhesion Cell 1992 69: 11–25
Bloch W et al. Beta-1 integrin is essential for teratoma growth and angiogenesis J Cell Biol 1997 139: 265–278
Yang JT, Rayburn H, Hynes RO . Embryonic mesodermal defects in alpha 5 integrin-deficient mice Development 1993 119: 1093–1105
Yang JT, Rayburn H, Hynes RO . Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development Development 1995 121: 549–560
Senger DR et al. Angiogenesis promoted by vascular endothelial growth factor – regulation through alpha(1)beta(1) and alpha(2)beta(1) integrins Proc Natl Acad Sci USA 1997 94: 13612–13617
Brooks PC . Role of integrins in angiogenesis Eur J Cancer 1996 14: 2423–2429
Drake CJ, Cheresh DA, Little CD . An antagonist of integrin alpha v beta 3 prevents maturation of blood vessels during embryonic neovascularization J Cell Sci 1995 108: 2655–2661
Bader BL, Rayburn H, Crowley D, Hynes RO . Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha-v integrins Cell 1998 95: 507–519
Brooks PC et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels Cell 1994 79: 1157–1164
Max R et al. Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas Int J Cancer 1997 71: 320–324
Brooks PC, Clark RA, Cheresh DA . Requirement of vascular integrin alpha v beta 3 for angiogenesis Science 1994 264: 569–571
Friedlander M et al. Definition of two angiogenic pathways by distinct alpha v integrins Science 1995 270: 1500–1502
Brooks PC et al. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin J Clin Invest 1995 96: 1815–1822
Stromblad S et al. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis J Clin Invest 1996 98: 426–433
Soldi R et al. Role of alpha(v)beta(3) integrin in the activation of vascular endothelial growth factor receptor-2 EMBO J 1999 18: 882–892
Brooks PC et al. Localization of matrix metalloproteinase mmp-2 to the surface of invasive cells by interaction with integrin alpha-v-beta-3 Cell 1996 85: 683–693
Brooks PC et al. Disruption of angiogenesis by pex, a noncatalytic metalloproteinase fragment with integrin binding activity Cell 1998 92: 391–400
Ruegg C et al. Evidence for the involvement of endothelial cell integrin alpha-v-beta-3 in the disruption of the tumor vasculature induced by tnf and ifn-gamma Nature Med 1998 4: 408–414
Dedhar S, Hannigan GE . Integrin cytoplasmic interactions and bidirectional transmembrane signalling Curr Opin Cell Biol 1996 8: 657–669
LaFlamme SE, Akiyama SK, Yamada KM . Regulation of fibronectin receptor distribution (published erratum appears in J Cell Biol 1992; 118: 491) J Cell Biol 1992 117: 437–447
LaFlamme SE, Thomas LA, Yamada SS, Yamada KM . Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly J Cell Biol 1994 126: 1287–1298
Smilenov L, Briesewitz R, Marcantonio EE . Integrin beta 1 cytoplasmic domain dominant negative effects revealed by lysophosphatidic acid treatment Molec Biol Cell 1994 5: 1215–1223
Lukashev ME, Sheppard D, Pytela R . Disruption of integrin function and induction of tyrosine phosphorylation by the autonomously expressed beta 1 integrin cytoplasmic domain J Biol Chem 1994 269: 18311–18314
Korhonen J et al. Endothelial-specific gene expression directed by the tie gene promoter in vivo Blood 1995 86: 1828–1835
Iljin K et al. Role of Ets factors in the activity and endothelial cell specificity of the mouse Tie gene promoter FASEB J 1999 13: 377–386
Re F et al. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells J Cell Biol 1994 127: 537–546
Frisch SM, Francis H . Disruption of epithelial cell–matrix interactions induces apoptosis J Cell Biol 1994 124: 619–626
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C . A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V J Immunol Methods 1995 184: 39–51
Idziorek T, Estaquier J, De Bels F, Ameisen JC . YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability J Immunol Methods 1995 185: 249–258
Luque A et al. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain J Biol Chem 1996 271: 11067–11075
Mastrangelo AM, Homan SM, Humphries MJ, LaFlamme SE . Amino acid motifs required for isolated beta cytoplasmic domains to regulate ‘in trans’ beta1 integrin conformation and function in cell attachment J Cell Sci 1999 112: 217–229
Diaz-Gonzalez F, Forsyth J, Steiner B, Ginsberg MH . Trans-dominant inhibition of integrin function Molec Biol Cell 1996 7: 1939–1951
Filardo EJ et al. Requirement of the NPXY motif in the integrin beta 3 subunit cytoplasmic tail for melanoma cell migration in vitro and in vivo J Cell Biol 1995 130: 441–450
Ylanne J et al. Mutation of the cytoplasmic domain of the integrin beta 3 subunit. Differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis J Biol Chem 1995 270: 9550–9557
Laflamme SE, Homan SM, Bodeau AL, Mastrangelo AM . Integrin cytoplasmic domains as connectors to the cells signal transduction apparatus Matrix Biol 1997 16: 153–163
Tahiliani PD, Singh L, Auer KL, LaFlamme SE . The role of conserved amino acid motifs within the integrin beta3 cytoplasmic domain in triggering focal adhesion kinase phosphorylation J Biol Chem 1997 272: 7892–7898
Liu KY, Timmons S, Lin YZ, Hawiger J . Identification of a functionally important sequence in the cytoplasmic tail of integrin beta 3 by using cell-permeable peptide analogs Proc Natl Acad Sci USA 1996 93: 11819–11824
Zimrin AB et al. Structure of platelet glycoprotein IIIa. A common subunit for two different membrane receptors J Clin Invest 1988 81: 1470–1475
Schaack J, Langer S, Guo X . Efficient selection of recombinant adenoviruses by vectors that express beta-galactosidase J Virol 1995 69: 3920–3923
Becker T et al. Use of recombinant adenovirus for metabolic engineering of mammalian cells. In: MG Roth (ed.) Methods in Cell Biology vol.43: Academic Press: San Diego pp 161–189
Acknowledgements
The authors wish to thank Drs M Lukashev, D Reed, V Jongeneel, R Iggo and D Rimoldi for providing reagents and cells, Mr G Bieler, O Dormond and Mrs C Paroz for technical assistance. Dr K M Müller is acknowledged for critical reading of the manuscript, and Dr F Lejeune for continuous support. This work was supported by grants from the Swiss National Science Foundation (31–52946–97 to CR and DO) and by the BCV Foundation. CR is recipient of a SCORE A fellowship award from the Swiss National Science Foundation (32–41611.911).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oguey, D., George, P. & Rüegg, C. Disruption of integrin-dependent adhesion and survival of endothelial cells by recombinant adenovirus expressing isolated β integrin cytoplasmic domains. Gene Ther 7, 1292–1303 (2000). https://doi.org/10.1038/sj.gt.3301236
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301236