Abstract
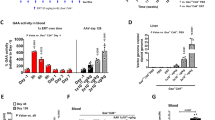
Pompe disease results from the deficiency of the lysosomal enzyme acid α-glucosidase (GAA), leading to accumulated glycogen in the heart and the skeletal muscles, which causes cardiomyopathy and muscle weakness. In this study, we tested the feasibility of gene therapy for Pompe disease using a lentivirus vector (LV). Newborn GAA knockout mice were treated with intravenous injection of LV encoding human GAA (hGAA) through the facial superficial temporal vein. The transgene expression in the tissues was analyzed up to 24 weeks after treatment. Our results showed that the recombinant LV was efficient not only in increasing the GAA activity in tissues but also in decreasing their glycogen content. The examination of histological sections showed clearence of the glycogen storage in skeletal and cardiac muscles 16 and 24 weeks after a single vector injection. Levels of expressed hGAA could be detected in serum of treated animals until 24 weeks. No significant immune reaction to transgene was detected in most treated animals. Therefore, we show that LV-mediated delivery system was effective in correcting the biochemical abnormalities and that this gene transfer system might be suitable for further studies on delivering GAA to Pompe disease mouse models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hirschhorn R, Reuser AJJ . Glycogen Storage Disease Type II (GSDII). McGraw-Hill: New York, 2001.
Raben N, Plotz P, Byrne BJ . Acid alpha-glucosidase deficiency (Glycogenosis type II, Pompe disease). Curr Mol Med 2002; 2: 145–166.
Kishnani PS, Howell R . Pompe disease in infants and children. J Pediatr 2004; 144: S35–S43.
Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D . A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 2006; 148: 671–675.
Van der Ploeg A, Reuser AJJ . Pompe's disease. Lancet 2008; 372: 1342–1353.
Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC-H, Waterson J et al. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 2006; 149: 89–97.
Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL et al. Recombinant human acid alpha-glucosidase. Major clinical benefits in infantile-onset Pompe disease. Neurology 2007; 68: 99–109.
Katzin LW, Amato AA . A review of the current diagnosis and treatment recommendations in the era of enzyme replacement therapy. J Clin Neuromuscul Dis 2008; 9: 421–431.
Ding E, Hu H, Hodges BL, Migone F, Serra D, Xu F et al. Efficacy of gene therapy for a prototypical lysosomal storage disease (GSD-II) is critically dependent on vector dose, transgene promoter, and the tissues targeted for vector transduction. Mol Ther 2002; 5: 436–446.
Fraites Jr TJ, Schleissing MR, Shanely RA, Walter GA, Cloutier DA, Zolotukhin I et al. Correction of the enzymatic and functional deficits in a model of Pompe disease using adeno-associated virus vectors. Mol Ther 2002; 5: 571–578.
Cresawn KO, Fraites Jr TJ, Wasserfall C, Atkinson M, Lewis M, Porvasnik S et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther 2005; 16: 68–80.
Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther 2005; 12: 876–884.
Sun B, Zhang H, Franco LM, Brown T, Bird A, Schneider A et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol Ther 2005; 11: 889–898.
Ziegler RJ, Bercury SD, Fidler J, Zhao MA, Foley J, Taksir TV et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther 2008; 19: 609–621.
Sun B, Young SP, Li P, Di C, Brown T, Salva MZ et al. Correction of multiple striated muscles in murine Pompe disease through adeno-associated virus-mediated gene therapy. Mol Ther 2008; 16: 1366–1371.
Mah C, Cresawn KO, Fraites Jr TJ, Pacak CA, Lewis MA, Zolotukhin I et al. Sustained correction of glycogen storage disease type II using adeno-associated virus serotype 1 vectors. Gene Therapy 2005; 12: 1405–1409.
Mah C, Pacak CA, Cresawn KO, DeRuisseau LR, Germain S, Lewis MA et al. Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors. Mol Ther 2007; 15: 501–507.
Biffi A, Naldini L . Gene therapy of storage disorders by retroviral and lentiviral vectors. Hum Gene Ther 2005; 16: 1133–1142.
Kobayashi H, Carbonaro D, Pepper K, Petersen D, Ge S, Jackson H et al. Neonatal gene therapy of MPS I mice by intravenous injection of a lentiviral vector. Mol Ther 2005; 11: 776–789.
Richard E, Douillard-Guilloux G, Batista L, Caillaud C . Correction of glycogenosis type 2 by muscle-specific lentiviral vector. In Vitro Cell Dev Biol Anim 2008; 44: 397–406.
Kimura T, Koya RC, Anselmi L, Sternini C, Wang H-J, Comin-Anduix B et al. Lentiviral vectors with CMV or MHCII promoters administered in vivo: immune reactivity versus persistence of expression. Mol Ther 2007; 15: 1390–1399.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Sun B, Bird A, Young SP, Kishnani PS, Chen Y-T, Koeberl DD . Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet 2007; 81: 1042–1049.
Ohashi T, Iizuka S, Ida H, Eto Y . Reduced alpha-Gal A enzyme activity in Fabry fibroblast cells and Fabry mice tissues induced by serum from antibody positive patients with Fabry disease. Mol Genet Metab 2008; 94: 313–318.
Cresawn KO, Fraites Jr TJ, Wasserfall C, Atkinson M, Lewis M, Porvasnik S et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther 2005; 16: 68–80.
Raben N, Nagaraju K, Lee A, Lu N, Rivera Y, Jatkar T et al. Induction of tolerance to a recombinant human enzyme, acid alpha-glucosidase, in enzyme deficient knockout mice. Transgenic Res 2003; 12: 171–178.
Joseph A, Munroe K, Housman M, Garman R, Richards S . Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin Exp Immunol 2008; 152: 138–146.
Ponder KP . Immunology of neonatal gene transfer. Curr Gene Ther 2007; 7: 403–410.
Ponder KP, Wang B, Wang P, Ma X, Herati R, Wang B et al. Mucopolysaccharidosis I cats mount a cytotoxic T lymphocyte response after neonatal gene therapy that can be blocked with CTLA4-Ig. Mol Ther 2006; 14: 5–13.
Daly TM, Vogler C, Levy B, Haskins ME, Sands MS . Neonatal gene transfer leads to widespread correction of pathology in a murine model of lysosomal storage disease. Proc Natl Acad Sci USA 1999; 96: 2296–2300.
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJE et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347.
Vigna E, Naldini L . Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J Gene Med 2000; 2: 308–316.
Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA 2006; 103: 17372–17377.
Di Domenico C, Di Napoli D, Gonzalez y Reyero E, Lombardo A, Naldini L, Di Natale P . Limited transgene immune response and long-term expression of human alpha-L-iduronidase in young adult mice with mucopolysaccharidosis type I by liver-directed gene therapy. Hum Gene Ther 2006; 17: 1112–1121.
McIntyre C, Roberts ALD, Ranieri E, Clements PR, Byers S, Anson DS . Lentiviral-mediated gene therapy for murine mucopolysaccharidosis type IIIA. Mol Genet Metab 2008; 93: 411–418.
Yoshimitsu M, Higuchi K, Ramsubir S, Nonaka T, Rasaiah VI, Siatskas C et al. Efficient correction of Fabry mice and patient cells mediated by lentiviral transduction of hematopoietic stem/progenitor cells. Gene Therapy 2007; 14: 256–265.
Douillard-Guilloux G, Richard E, Batista L, Caillaud C . Partial phenotipic correction and immune tolerance induction to enzyme replacement therapy after hematopoietic stem cell gene transfer of alpha-glucosidase in Pompe disease. J Gene Med 2009; 11: 279–287.
Krishnan M, Park JM, Cao F, Wang D, Paulmurugan R, Tseng JR et al. Effects of epigenetic modulation on reporter gene expression: implications for stem cell imaging. FASEB J 2006; 20: 106–108.
Fukuda T, Roberts A, Ahearn M, Zaal K, Ralston E, Plotz PH et al. Autophagy and lysosomes in Pompe disease. Autophagy 2006; 2: 318–320.
Fukuda T, Ewan L, Bauer M, Mattaliano RJ, Zaal K, Ralston E et al. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol 2006; 59: 700–708.
Raben N, Takikita S, Pittis MG, Bembi B, Marie SKN, Roberts A et al. Deconstructing Pompe disease by analyzing single muscle fibers. To see a world in a grain of sand. Autophagy 2007; 3: 546–552.
Raben N, Baum R, Schreiner C, Takikita S, Mizushima N, Ralston E et al. When more is less excess and deficiency of autophagy coexist in skeletal muscle in Pompe disease. Autophagy 2009; 5: 1–3.
Fukuda T, Ahearn M, Roberts A, Mattaliano RJ, Zaal K, Ralston E et al. Autophagy and mistargeting of therapeutic enzyme in skeletal muscle in Pompe disease. Mol Ther 2006; 14: 831–839.
Sun B, Zhang H, Bird A, Li S, Young SP, Koeberl DD . Impaired clearence of accumulated lysosomal glycogen in advanced Pompe disease despite high-level vector mediated transgene expression. J Gene Med 2009; 11: 913–920.
Raben N, Fukuda T, Gilbert AL, Jong D, Thurberg BL, Mattaliano RJ et al. Replacing acid alpha-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol Ther 2005; 11: 48–56.
Ding EY, Hodges BL, Hu H, McVie-Wylie AJ, Serra D, Migone FK et al. Long-term efficacy after [E1−, polymerase−] adenovirus-mediated transfer of human acid-alpha-glucosidase gene into glycogen storage disease type II knockout mice. Hum Gene Ther 2001; 12: 955–965.
Zhu Y, Li X, McVie-Wylie A, Jiang C, Thurberg BL, Raben N et al. Carbohydrate-remodelled acid á-glucosidase with higher affinity for the cation-independent mannose 6-phosphate receptor demonstrates improved delivery to muscles of Pompe mice. Biochem J 2005; 389: 619–628.
West LJ, Pollock-Barziv SM, Dipchand AI, Lee KJ, Cardella CJ, Benson LN et al. ABO-incompatible heart transplantation in infants. N Engl J Med 2001; 344: 793–800.
Chien Y-H, Chiang S-C, Zhang XK, Keutzer J, Lee N-C, Huang A-C et al. Early detection of Pompe disease by newborn screening is feasible: results from the Taiwan Screening Program. Pediatrics 2008; 122: e39–e45.
Madoiwa S, Yamauchi T, Hakamata Y, Kobayashi E, Arai M, Sugo T et al. Induction of immune tolerance by neonatal intravenous injection of human factor VIII in murine hemophilia A. J Thromb Haemost 2004; 2: 754–762.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 301: 415–419.
Stripecke R, Koya RC, Ta HQ, Kasahara N, Levine AM . The use of lentiviral vectors in gene therapy of leukemia: combinatorial gene delivery of immunomodulators into leukemia cells by state-of-the-art vectors. Blood Cells Mol Dis 2003; 31: 28–37.
Raben N, Nagaraju K, Lee E, Kessler P, Byrnei B, Lee L et al. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 1998; 273: 19086–19092.
Sands MS, Barker JE . Percutaneous intravenous injection in neonatal mice. Lab Anim Sci 1999; 49: 328–330.
Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther 2005; 11: 57–65.
Acknowledgements
We acknowledge Dr Y-T Chen (Department of Pediatrics, Duke University Medical Center, Durham, NC, USA) for providing the GAA cDNA, Dr N Kasahara (Department of Medicine, Division of Digestive Diseases, David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA, USA) for the generating lentiviral system, Dr N Raben (Arthritis and Rheumatism Branch, NIAMS, National Institutes of Health, Bethesda, MD, USA) for the Gaa−/− mice and the Genzyme Corporation for monoclonal anti-GAA antibodies. We also would like to express our thanks to our colleagues of the Department of Gene Therapy and to the staff of the Animal Facilities Division. Dr Kyosen was supported by a research fellowship from the Japan International Cooperation Agency (JICA) and the Uehara Foundation, Japan. This research was supported by The Jikei University Research Fund, received grants for The Research on Measures for Intractable Diseases, Japanese Ministry of Health Welfare and Labor, from Japan International Cooperation Agency (JICA) and Uehara Foundation-Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The department of Genetic Diseases of the Jikei University School of Medicine receives grants from the Genzyme Corporation.
Rights and permissions
About this article
Cite this article
Kyosen, S., Iizuka, S., Kobayashi, H. et al. Neonatal gene transfer using lentiviral vector for murine Pompe disease: long-term expression and glycogen reduction. Gene Ther 17, 521–530 (2010). https://doi.org/10.1038/gt.2009.160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.160
Keywords
This article is cited by
-
Molecular Approaches for the Treatment of Pompe Disease
Molecular Neurobiology (2020)
-
Case study: monitoring of Glc4 tetrasaccharide in the urine of Pompe patients, use of MALDI-TOF MS, and 1H NMR
Chemical Papers (2019)
-
Pompe Disease: From Basic Science to Therapy
Neurotherapeutics (2018)
-
The humanistic burden of Pompe disease: are there still unmet needs? A systematic review
BMC Neurology (2017)
-
TFEB overexpression promotes glycogen clearance of Pompe disease iPSC-derived skeletal muscle
Molecular Therapy - Methods & Clinical Development (2016)